Page 28 - Adsorbents fundamentals and applications
P. 28
BASIC CONSIDERATIONS FOR SORBENT DESIGN 13
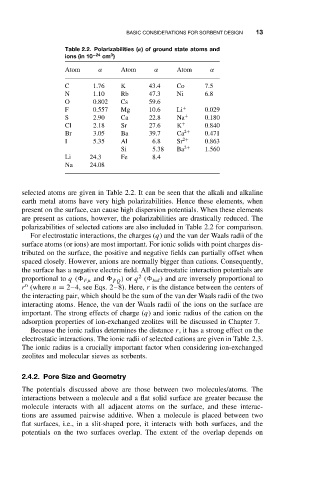
Table 2.2. Polarizabilities (α) of ground state atoms and
3
ions (in 10 −24 cm )
Atom α Atom α Atom α
C 1.76 K 43.4 Co 7.5
N 1.10 Rb 47.3 Ni 6.8
O 0.802 Cs 59.6
F 0.557 Mg 10.6 Li + 0.029
S 2.90 Ca 22.8 Na + 0.180
Cl 2.18 Sr 27.6 K + 0.840
Br 3.05 Ba 39.7 Ca 2+ 0.471
I 5.35 Al 6.8 Sr 2+ 0.863
Si 5.38 Ba 2+ 1.560
Li 24.3 Fe 8.4
Na 24.08
selected atoms are given in Table 2.2. It can be seen that the alkali and alkaline
earth metal atoms have very high polarizabilities. Hence these elements, when
present on the surface, can cause high dispersion potentials. When these elements
are present as cations, however, the polarizabilities are drastically reduced. The
polarizabilities of selected cations are also included in Table 2.2 for comparison.
For electrostatic interactions, the charges (q) and the van der Waals radii of the
surface atoms (or ions) are most important. For ionic solids with point charges dis-
tributed on the surface, the positive and negative fields can partially offset when
spaced closely. However, anions are normally bigger than cations. Consequently,
the surface has a negative electric field. All electrostatic interaction potentials are
2
) or q ( Ind ) and are inversely proportional to
proportional to q( Fµ and ˙ FQ
n
r (where n = 2–4, see Eqs. 2–8). Here, r is the distance between the centers of
the interacting pair, which should be the sum of the van der Waals radii of the two
interacting atoms. Hence, the van der Waals radii of the ions on the surface are
important. The strong effects of charge (q) and ionic radius of the cation on the
adsorption properties of ion-exchanged zeolites will be discussed in Chapter 7.
Because the ionic radius determines the distance r, it has a strong effect on the
electrostatic interactions. The ionic radii of selected cations are given in Table 2.3.
The ionic radius is a crucially important factor when considering ion-exchanged
zeolites and molecular sieves as sorbents.
2.4.2. Pore Size and Geometry
The potentials discussed above are those between two molecules/atoms. The
interactions between a molecule and a flat solid surface are greater because the
molecule interacts with all adjacent atoms on the surface, and these interac-
tions are assumed pairwise additive. When a molecule is placed between two
flat surfaces, i.e., in a slit-shaped pore, it interacts with both surfaces, and the
potentials on the two surfaces overlap. The extent of the overlap depends on