Page 77 - Adsorbents fundamentals and applications
P. 77
62 PORE SIZE DISTRIBUTION
Sorbent Adsorbate layer Sorbent
surface surface
L
d S d S
d A
d 0 d 0
(L − d )
S
Free space
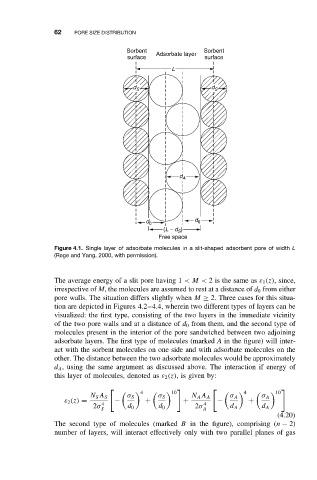
Figure 4.1. Single layer of adsorbate molecules in a slit-shaped adsorbent pore of width L
(Rege and Yang, 2000, with permission).
The average energy of a slit pore having 1 <M < 2is the same as ε 1 (z), since,
irrespective of M, the molecules are assumed to rest at a distance of d 0 from either
pore walls. The situation differs slightly when M ≥ 2. Three cases for this situa-
tion are depicted in Figures 4.2–4.4, wherein two different types of layers can be
visualized: the first type, consisting of the two layers in the immediate vicinity
of the two pore walls and at a distance of d 0 from them, and the second type of
molecules present in the interior of the pore sandwiched between two adjoining
adsorbate layers. The first type of molecules (marked A in the figure) will inter-
act with the sorbent molecules on one side and with adsorbate molecules on the
other. The distance between the two adsorbate molecules would be approximately
d A , using the same argument as discussed above. The interaction if energy of
this layer of molecules, denoted as ε 2 (z), is given by:
4 10 4 10
N S A S σ S σ S N A A A σ A σ A
ε 2 (z) = 4 − + + 4 − +
2σ d 0 d 0 2σ d A d A
S A
(4.20)
The second type of molecules (marked B in the figure), comprising (n − 2)
number of layers, will interact effectively only with two parallel planes of gas