Page 78 - Adsorbents fundamentals and applications
P. 78
HORV ´ ATH–KAWAZOE APPROACH 63
Sorbent Sorbent
surface Adsorbate layers surface
L
A A d S
d A
d A
d 0 d 0
(L − d )
S
Free space
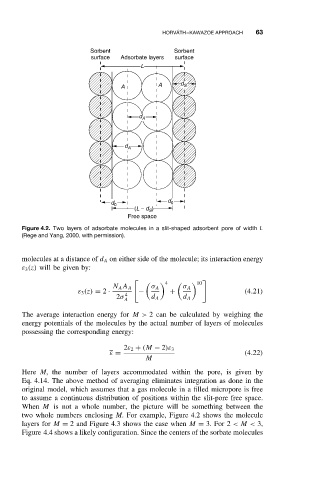
Figure 4.2. Two layers of adsorbate molecules in a slit-shaped adsorbent pore of width L
(Rege and Yang, 2000, with permission).
molecules at a distance of d A on either side of the molecule; its interaction energy
ε 3 (z) will be given by:
4 10
N A A A σ A σ A
ε 3 (z) = 2 · − + (4.21)
2σ 4 d A d A
A
The average interaction energy for M> 2 can be calculated by weighing the
energy potentials of the molecules by the actual number of layers of molecules
possessing the corresponding energy:
2ε 2 + (M − 2)ε 3
ε = (4.22)
M
Here M, the number of layers accommodated within the pore, is given by
Eq. 4.14. The above method of averaging eliminates integration as done in the
original model, which assumes that a gas molecule in a filled micropore is free
to assume a continuous distribution of positions within the slit-pore free space.
When M is not a whole number, the picture will be something between the
two whole numbers enclosing M. For example, Figure 4.2 shows the molecule
layers for M = 2 and Figure 4.3 shows the case when M = 3. For 2 <M < 3,
Figure 4.4 shows a likely configuration. Since the centers of the sorbate molecules