Page 89 - Adsorbents fundamentals and applications
P. 89
74 PORE SIZE DISTRIBUTION
3.0 Original HK
Original HK–CY
2.5
Modified HK
2.0 Modified HK–CY
d(W/W 0 )/dL 1.5
1.0
0.5
0.0
8 10 12 14 16 18 20
Effective cavity size (Å)
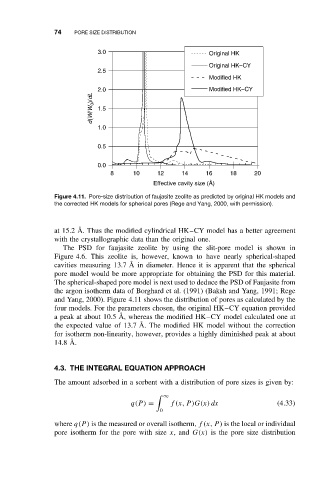
Figure 4.11. Pore-size distribution of faujasite zeolite as predicted by original HK models and
the corrected HK models for spherical pores (Rege and Yang, 2000, with permission).
at 15.2 ˚ A. Thus the modified cylindrical HK–CY model has a better agreement
with the crystallographic data than the original one.
The PSD for faujasite zeolite by using the slit-pore model is shown in
Figure 4.6. This zeolite is, however, known to have nearly spherical-shaped
cavities measuring 13.7 ˚ A in diameter. Hence it is apparent that the spherical
pore model would be more appropriate for obtaining the PSD for this material.
The spherical-shaped pore model is next used to deduce the PSD of Faujasite from
the argon isotherm data of Borghard et al. (1991) (Baksh and Yang, 1991; Rege
and Yang, 2000). Figure 4.11 shows the distribution of pores as calculated by the
four models. For the parameters chosen, the original HK–CY equation provided
a peak at about 10.5 ˚ A, whereas the modified HK–CY model calculated one at
the expected value of 13.7 ˚ A. The modified HK model without the correction
for isotherm non-linearity, however, provides a highly diminished peak at about
14.8 ˚ A.
4.3. THE INTEGRAL EQUATION APPROACH
The amount adsorbed in a sorbent with a distribution of pore sizes is given by:
∞
q(P ) = f (x, P )G(x) dx (4.33)
0
where q(P ) is the measured or overall isotherm, f(x, P ) is the local or individual
pore isotherm for the pore with size x,and G(x) is the pore size distribution