Page 84 - Adsorbents fundamentals and applications
P. 84
HORV ´ ATH–KAWAZOE APPROACH 69
model. The total number of concentric layers of adsorbate molecules that can
be accommodated in the cylinder of radius L is first determined. The energy
potential of the adsorbate molecules in each layer is then calculated by using the
appropriate equation corresponding to that given by Everett and Powl (1976).
The number of sorbate molecules in each sorbate layer is estimated by using the
sorbate molecule diameter, and the diameter of the corresponding concentric lay-
ers. The average interaction energy is then determined by a population-weighted
average of the individual layer potentials. One of the assumptions made by the
model is that because adsorption will proceed from the extreme periphery of the
pore towards the center, the energy potential of each layer would correspond to
only the field induced by the immediately surrounding outer layer of molecules.
The interaction energy induced by the inside layer of molecules, enclosed by the
molecules for which the energy potential is being calculated, is considered to
be negligible.
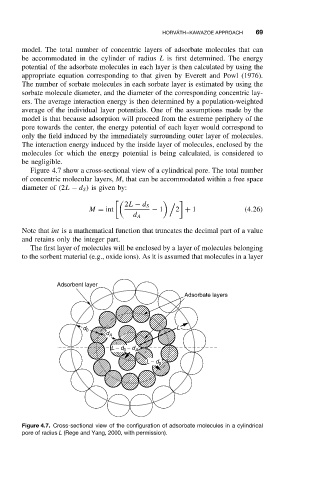
Figure 4.7 show a cross-sectional view of a cylindrical pore. The total number
of concentric molecular layers, M, that can be accommodated within a free space
diameter of (2L − d S ) is given by:
à
2L − d S
M = int − 1 2 + 1 (4.26)
d A
Note that int is a mathematical function that truncates the decimal part of a value
and retains only the integer part.
The first layer of molecules will be enclosed by a layer of molecules belonging
to the sorbent material (e.g., oxide ions). As it is assumed that molecules in a layer
Adsorbent layer
Adsorbate layers
d 0 L
d A
L − d − d A
0
L − d 0
Figure 4.7. Cross-sectional view of the configuration of adsorbate molecules in a cylindrical
pore of radius L (Rege and Yang, 2000, with permission).