Page 100 - Adsorption by Powders and Porous Solids
P. 100
CHAPTER 3. METHODOLOGY OF ADSORPTION 85
described just above, but it may allow the balance to be used over a smaller range,
where a higher sensitivity is available.
A pertinent question is: as the'volume of the adsorbed phase increases, do we have to
&e into account the corresponding increase of buoyancy? (e.g. the buoyancy doubles
after saturation of an adsorbent with 50% porosity). The answer is no, provided we want
U, asess the surface excess mass mu. As illustrated in Figure 3.22, because of the buoy-
mq effect, we do not measure the total mass of the adsorbed layer (shaded + hatched
m) but simply a surface excess mass (hatched area only). Thus, adsorption gravime-
and the Gibbs representation are highly compatible (Findenegg, 1997).
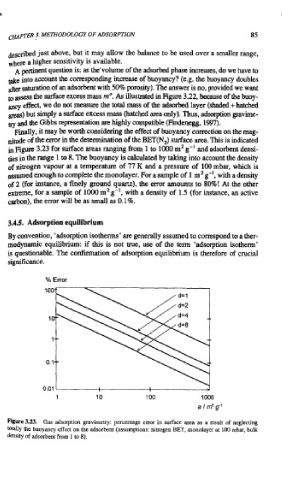
Finally, it may be worth considering the effect of buoyancy correction on the mag-
nitude of the error in the determination of the BET(NJ surface area. This is indicated
in Figure 3.23 for surface areas ranging from 1 to 1000 mZ g-' and adsorbent densi-
ties in the range 1 to 8. The buoyancy is calculated by taking into account the density
of nitrogen vapour at a temperature of 77 K and a pressure of 100 mbar, which is
assumed enough to complete the monolayer. For a sample of 1 mZ g-', with a density
of 2 (for instance, a finely ground quartz), the error amounts to 80%! At the other
extreme, for a sample of 1000 m2 g-', with a density of 1.5 (for instance, an active
carbon), the error will be as small as 0.1%.
3.4.5. Adsorption equilibrium
By convention, 'adsorption isotherms' are generally assumed to correspond to a ther-
modynamic equilibrium: if this is not true, use of the term 'adsorption isotherm'
is questionable. The confiiation of adsorption equilibrium is the~efore of crucial
significance.
% Error
Figure 3.23. Gas adsorption gravimetry: percentage error in surface area as a result of neglecting
totally the buoyancy effect on the adsorbenr (assumptions: nitrogen BET, monolayer at 100 rnbar, bulk
density of adsorbent from 1 to 8).