Page 198 - Adsorption by Powders and Porous Solids
P. 198
C~~pTER ASSESSMENT OF SURFACE AREA 185
6.
sents problems when it is associated with the BET theory. In contrast, an FHH-
gd treatment appears to be more promising, provided that wetting effects are taken
into account. This was exactly the approach adopted by Krim and her co-workers.
their work on carefully prepared homogeneous silver films, Krim and Panella
(1991) and Panella and Krim (1994) applied the FHH equation in the form
where the coefficient a is dependent on the nature of the adsorbent-adsorbate and
ads~rbate-adsorbate interactions and the corresponding fractal dimension is deter-
&ed by the magnitude of the exponent n (de Gennes, 1985). Nitrogen and oxygen
isotherms at 77.4 K gave linear FHH plots over a wide range of multilayer coverage.
fie values of n for nitrogen on smooth and rough silver substrates were 3.0 and 4.7,
respectively, the former giving the expected fractal dimension, D, = 2. However, the
"due n = 3 was also obtained for oxygen on the rough surface, which was attributed
to surface tension effects (since the surface tension of liquid oxygen is much higher
than that of liquid nitrogen).
Panella and Krim suggested that the high value n = 4.7 was more consistent with
the properties of a self-affine surface rather than a self-similar one. Self-affi~le frac-
tals are associated with asymmetric scaling, that is different scaling relations in
different directions (Avnir, 1997).
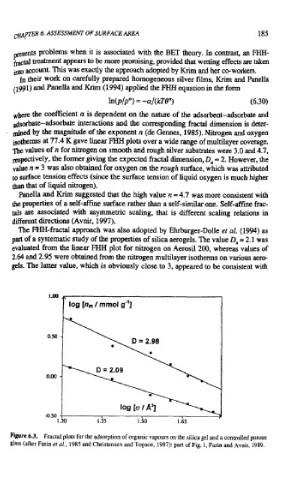
The FHH-fractal approach was also adopted by Ehrburger-Dolle et al, (1994) as
part of a systematic study of the properties of silica aerogels. The value D, = 2.1 was
evaluated from the linear FHH plot for nitrogen on Aerosil200, whereas values of
2.64 and 2.95 were obtained from the nitrogen multilayer isotherms on various aero-
gels. The latter value, which is obviously close to 3, appeared to be consistent with
1.00
log [n, / mmoi g-'1
log [o A2]
/
-0.50 , 1
I .20 1.35 1 .SO 1.65
Figure 6.3. Fractal plots for the adsorption of organic vapours on the silica gel and a controlled porous
glass (after Fain er al., 1985 and Christensen and Topsoe, 1987): part of Fig. 1, Farin and Avnir, 1989.