Page 257 - Adsorption by Powders and Porous Solids
P. 257
9. ADSORPTION BY ACTIVE CARBONS 245
could serve as standard for both graphitized and non-graphitized carbons. There are
three main reasons why there are significant discrepancies between proposed stan-
dard data to be found in the literature. First, any significant differences in the
pphitic surface structure will have some effect on the isotherm shape - especially
low surface coverage. Second, any interparticle capillary condensation will
an upward deviation in the multilayer/capillary condensation region. Third,
any microporosity will enhance the adsorption in the sub-monolayer region and will
tend also to reduce the isothenn slope in the multilayer region.
As explained in Chapters 6 and 8, by applying the as-method we have a simple
way of checking the validity of the BET area and detecting the presence of micro-
porosiv. Many carbon blacks have been found to be essentially non-microporous
(Cmott et al., 1987; Bradley et al., 1995), in which case the corresponding values of
BET area and as area are in good agreement. However, in a few cases the back-
of the as-plot has given a positive intercept on the adsorption axis
which is an indication of some microporosity. The microporous nature of some
carbon blacks has been confmed in several recent investigations (Stoeckli et al.,
1994a; Kruk et al., 1996). As one might expect, oxidation leads to a considerable
increase in the level of the microporosity (Bradley et al., 1995).
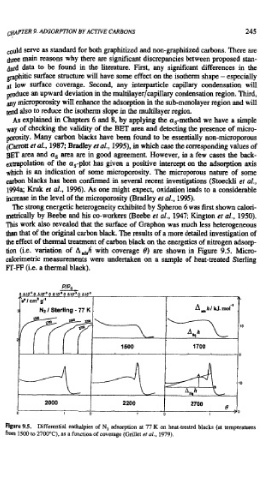
The strong energetic heterogeneity exhibited by Spheron 6 was first shown calori-
metrically by Beebe and his co-workers (Beebe el al., 1947; Kington et al., 1950).
Tbis work also revealed that the surface of Graphon was much less heterogeneous
than that of the original carbon black. The results of a more detailed investigation of
the effect of thermal treatment of carbon black on the energetics of nitrogen adsorp-
tion (i.e. variation of ~,h with coverage 19) are shown in Figure 9.5. Micro-
calorimetric measurements were undertaken on a sample of heat-treated Sterling
FT-FF (i.e. a thermal black).
-
PIPo
0
o 5.1~~ 5.1~' o 6.70-' o 5.10~ o S.~O-~
A
I
Ag em' g"
3 Nz I Sterling - 77 K . A,h/ kl.moil ,
lo
2
1500 1700
0
* 4
7-44-
10
2000 2200 liqh
2700
0 I 0 1 0 1 @ $0
Figure 9.5. Differential enthalpies of N, adsorption at 77 K on heat-treated blacks (at temperatures
from 1500 to 2700°C). as a function of coverage (Grillet er al.. 1979).