Page 260 - Adsorption by Powders and Porous Solids
P. 260
248 ADSORPTION BY POWDERS AND POROUS SOms
The energetic heterogeneity of the original ungraphitized surface was revealed by
the careful adsorption calorimetric measurements undertaken by Beebe et al., (1953).
By comparing the changes in the differential energies of adsorption for argon on
Spheron and Graphon, these investigators found that the initial steep decline in 'dif-
ferential heat of adsorption' was largely removed as a result of graphitization.
Instead, an increase in the differential energy was observed at a higher surface cov-
erage: it is now generally agreed that this was due to the adsorbate-adsorbate inter-
action becoming apparent as the degree of energetic heterogeneity was reduced.
A systematic study of laypton adsorption on exfoliated graphite was subsequently
undertaken by Thomy and co-workers (Thorny and Duval, 1969; Thorny et a!.,
1972). Their stepwise isotherm, determined at 77.3 K, is shown in Figure 4.1. The
layer-by-layer nature of the physisorption process is clearly evident - at least up to
four molecular layers. This isotherm shape is remarkably similar to that of the
krypton isotherm on graphitized carbon black reported by Arnberg er al., (1955).
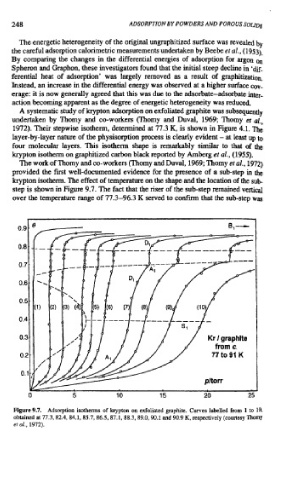
The work of Thorny and co-workers (Thorny and Duval, 1969; Thorny et al., 1972)
provided the first well-documented evidence for the presence of a sub-step in the
krypton isotherm. The effect of temperature on the shape and the location of the sub-
step is shown in Figure 9.7. The fact that the riser of the sub-step remained vertical
over the temperature range of 77.3-96.3 K served to confirm that the sub-step was
Figure 9.7. Adsorption isotherms of krypton on exfoliated graphite. Curves labelled from 1 to 10,
obtained at 77.3, 82.4, 84.1,85.7,86.5,87.1, 88.3.89.0.90.1 and 90.9 K, respectively (courtesy Thorny
eta[., 1972).