Page 306 - Adsorption by Powders and Porous Solids
P. 306
CHAPTER 10. ADSORPTION BY METAL OXIDES
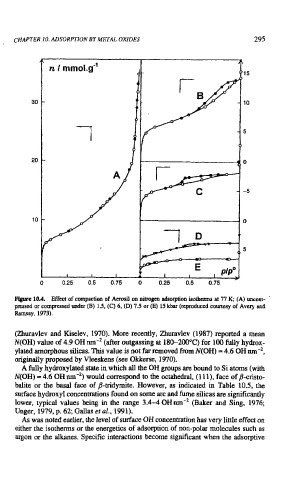
Fire 10.4. Effect of compaction of Aerosil on nitrogen adsorption isotherms at 77 K; (A) uncorn- '
pressed or compressed under (B) 1.5, (C) 6, (D) 7.5 or (E) 15 kbar (reproduced courtesy of Avery and
Ramsay, 1973).
(Zhuravlev and Kiselev, 1970). More recently, Zhuravlev (1987) reported a mean
N(0H) value of 4.9 OH nm-2 (after outgassing at 180-200°C) for 100 llly hydrox-
ylated amorphous silicas. This value is not far removed from N(0H) = 4.6 OH nrn-2,
originally proposed by Vleeskens (see Okkerse, 1970).
A fully hydroxylated state in which all the OH groups are bound to Si atoms (with
N(0H) = 4.6 OH nrn-2) would correspond to the octahedral, (1 1 I), face of B-cristo-
balite or the basal face of B-tridymite. However, as indicated in Table 10.5, the
surface hydroxyl concentrations found on some arc and fume silicas are significantly
lower, typical values being in the range 3.4-4 OHII~-~ (Baker and Sing, 1976;
Unger, 1979, p. 62; Gallas et al., 1991).
As was noted earlier, the level of surface OH concentration has very little effect on
either the isotherms or the energetics of adsorption of non-polar molecules such as
argon or the alkanes. Specific interactions become significant when the adsorptive