Page 340 - Adsorption by Powders and Porous Solids
P. 340
CWER 10. ADSORPTION BY METAL OXIDES
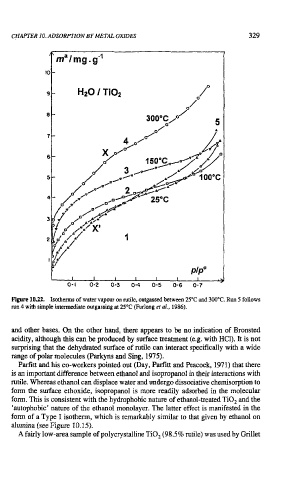
Figure 10.22. Isotherms of water vapour on nrtile, outgassed between 25°C and 300°C. Run 5 follows
run 4 with simple intermediate outgassing at 25OC (Furlong et al., 1986).
and other bases. On the other hand, there appears to be no indication of Bronsted
acidity, although this can be produced by surface treatment (e.g. with HCI). It is not
surprising that the dehydrated surface of rutile can interact specifically with a wide
range of polar molecules (Parkyns and Sing, 1975).
Parfitt and his co-workers pointed out (Day, Parfitt and Peacock, 1971) that there
is an important difference between ethanol and isopropanol in their interactions with
rutile. Whereas ethanol can displace water and undergo dissociative chemisorption to
form the surface ethoxide, isopropanol is more readily adsorbed in the molecular
form. This is consistent with the hydrophobic nature of ethanol-treated TiO, and the
'autophobic' nature of the ethanol monolayer. The latter effect is manifested in the
form of a Type I isotherm, which is remarkably similar to that given by ethanol on
alumina (see Figure 10.15).
A fairly low-area sample of polycrystalline TiO, (98.5% rutile) was used by Grillet