Page 345 - Adsorption by Powders and Porous Solids
P. 345
334 ADSORPTION BY POWDERS MD POROUS SOLIDS
Vilches, 1984). The deleterious effect of water vapour was attributed to the formation
of surface layers of Mg(OH),. This conclusion was consistent with the inferior
quality (i.e. non-stepwise character) of isotherms determined on MgO prepared by
the thermal decomposition of Mg(OH),.
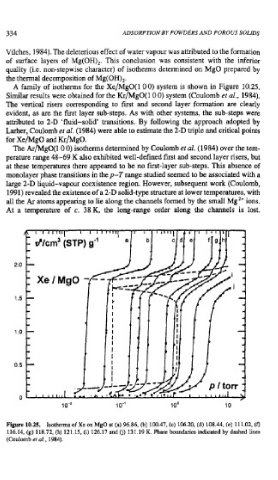
A family of isotherms for the Xe/MgO(l 0 0) system is shown in Figure 10.25.
Similar results were obtained for the Kr/MgO(l 0 0) system (Coulomb et al., 1984).
The vertical risers corresponding to first and second layer formation are clearly
evident, as are the first layer sub-steps. As with other systems, the sub-steps were
attributed to 2-D 'fluid-solid' transitions. By following the approach adopted by
Larher, Coulomb et al. (1984) were able to estimate the 2-D triple and critical points
for Xe/MgO and Kr/MgO.
The Ar/MgO(l 0 0) isotherms determined by Coulomb et al. (1984) over the tem-
perature range 48-69 K also exhibited well-defined first and second layer risers, but
at these temperatures there appeared to be no first-layer sub-steps. This absence of
monolayer phase transitions in thep-T range studied seemed to be associated with a
large 2-D liquid-vapour coexistence region. However, subsequent work (Coulomb,
1991) revealed the existence of a 2-D solid-type structure at lower temperatures, with
all the Ar atoms appearing to lie along the channels formed by the small ~g" ions.
At a temperature of c. 38 K, the long-range order along the channels is lost.
Figure 10.25. Isotherms of Xe on MgO at (a) 96.86, (b) 100.47, (c) 106.20, (d) 108.44, (e) 11 1.02, (f)
11 6.14, (g) 118.72, (h) 12 1.15, (i) 126.17 and Cj) 13 1.19 K. Phase boundaries indicated by dashed lines
(Coulomb et al., 1984).