Page 222 - Adsorption, Ion Exchange & Catalysis- 2007, Elsevier - Copy
P. 222
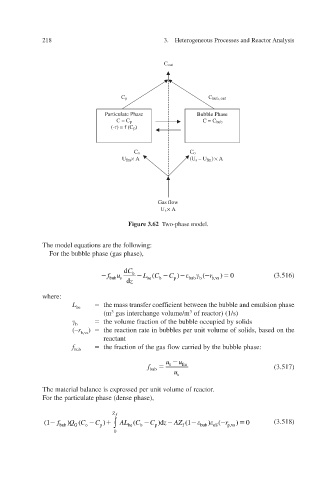
Else_AIEC-INGLE_cH003.qxd 7/13/2006 1:46 PM Page 218
218 3. Heterogeneous Processes and Reactor Analysis
C out
C p C bub, out
Particulate Phase Bubble Phase
C = C p C = C bub
(-r) = f (C p )
C o C o
U fm × A (U s – U fm ) × A
Gas flow
U s × A
Figure 3.62 Two-phase model.
The model equations are the following:
For the bubble phase (gas phase),
C d b
f bub s u L be C ( b C ) b,vs r ( ) 0 (3.516)
p
bub b
z d
where:
L the mass transfer coef ubble and emulsion phase f icient between the b
be
(m 3 gas interchange v olume/m 3 of reactor) (1/s)
the volume fraction of the bubble occupied by solids
b
(– r ) the reaction rate in bubbles per unit volume of solids, based on the
b,vs
reactant
f the fraction of the gas flow carried by the bubble phase:
bub
u u
f bub s fm (3.517)
u
s
The material balance is expressed per unit volume of reactor .
For the particulate phase (dense phase),
Z f
(
(1 f ) Q C C ) AL C be∫ (C z AZ )d (1 )r ( ) 0 (3.518)
bub G o p b p f bub eS p,vs
0