Page 272 - Adsorption, Ion Exchange & Catalysis- 2007, Elsevier - Copy
P. 272
Else_AIEC-INGLE_cH004.qxd 7/1/2006 6:53 PM Page 268
268 4. Adsorption and Ion Exchange
where
K F Q k M (4.15)
Applying eq. (4.13) for C e = C , o
q
max kC Fr (1) (4.16)
Q M o
Here, q max is the solid-phase concentration in equilibrium with C . Di o viding eqs. (4.13) and
(4.16),
Y X Fr (4.17)
It is important to distinguish between the values of q max and Q M . The first is the solid-phase
concentration in equilibrium with the initial fluid-phase concentration, while Q M is higher,
representing the maximum adsorption capacity, which is typically achieved in higher fluid-
phase concentrations. Fwing the terminology of Inglezakis (2005), for ion e xchange
ollo
q max corresponds to the maximum exchange level (MEL), while Q M corresponds to the real
exchange capacity (REC).
The empirical constants La and Fr are related to the particular system under in estiga- v
tion and are obtained from laboratory experiments (Chen et al ., 1968; Chern and Chien,
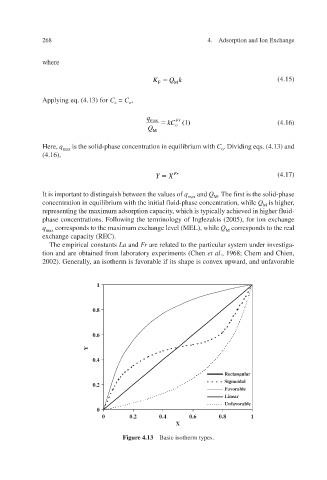
2002). Generally, an isotherm is forable if its shape is con and unf a v ard, x upw v e a orable v
1
0.8
0.6
Y
0.4
Rectangular
Sigmoidal
0.2
Favorable
Linear
Unfavorable
0
0 0.2 0.4 0.6 0.8 1
X
Figure 4.13 Basic isotherm types.