Page 267 - Adsorption, Ion Exchange & Catalysis- 2007, Elsevier - Copy
P. 267
Else_AIEC-INGLE_cH004.qxd 7/1/2006 6:53 PM Page 263
4.1 Basic Principles of Adsorption and Ion Exchange 263
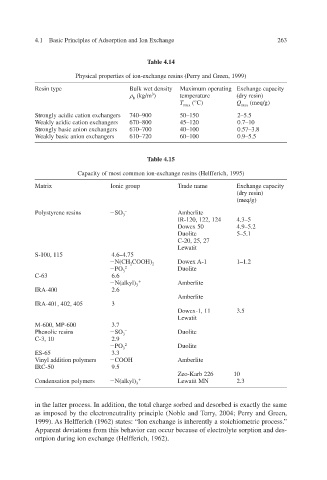
Table 4.14
Physical properties of ion-exchange resins (Perry and Green, 1999)
Resin type Bulk wet density Maximum operating Exchange capacity
(kg/m 3 ) temperature (dry resin)
b
T max (°C) Q max (meq/g)
Strongly acidic cation e xchangers 740–900 50–150 2–5.5
Weakly acidic cation exchangers 670–800 45–120 0.7–10
xchangers Strongly basic anion e 670–700 40–100 0.57–3.8
Weakly basic anion exchangers 610–720 60–100 0.9–5.5
Table 4.15
Capacity of most common ion-exchange resins (Helf 1995) ferich,
Matrix Ionic group Trade name Exchange capacity
(dry resin)
(meq/g)
Polystyrene resins SO 3 – Amberlite
IR-120, 122, 124 4.3–5
Dowex 50 4.9–5.2
Duolite 5–5.1
C-20, 25, 27
Lewatit
S-100, 115 4.6–4.75
N(CH 2 COOH) 2 Dowex A-1 1–1.2
PO 3 2– Duolite
C-63 6.6
yl) N(alk 3 Amberlite
IRA-400 2.6
Amberlite
IRA-401, 402, 405 3
Dowex-1, 11 3.5
Lewatit
M-600, MP-600 3.7
Phenolic resins SO 3 – Duolite
C-3, 10 2.9
PO 3 2– Duolite
ES-65 3.3
Vinyl addition polymers COOH Amberlite
IRC-50 9.5
Zeo-Karb 226 10
Condensation polymers yl) N(alk 3 Lewatit MN 2.3
in the latter process. In addition, the total charge sorbed and desorbed is exactly the same
,
erry
as imposed by the electroneutrality principle (Noble and T 2004; Perry and Green,
1999). As Helfferich (1962) states: “Ion exchange is inherently a stoichiometric process. ”
Apparent deviations from this behavior can occur because of electrolyte sorption and des-
ferich, ortpion during ion exchange (Helf 1962).