Page 265 - Adsorption, Ion Exchange & Catalysis- 2007, Elsevier - Copy
P. 265
Else_AIEC-INGLE_cH004.qxd 7/1/2006 6:53 PM Page 261
4.1 Basic Principles of Adsorption and Ion Exchange 261
Table 4.13
Chelating cation resin relativities for metal ions e selecti v
(relative selectivity is based on Ca 2 )
Metal ion Relative selectivity
Hg 2 2800
Cu 2 2300
Pb 2 1200
Ni 2 57
Zn 2 17
Cd 2 15
Co 2 6.7
Fe 2 4.7
Mn 2 1.2
Ca 2 1
example, the preference for mercury is 2800 times that for calcium. This means that in the
treatment of a solution, which contains equal molar concentrations of mercury and calcium
ions, the molar concentration of mercury ions on the resin will be 2800 times that of cal-
cium ions.
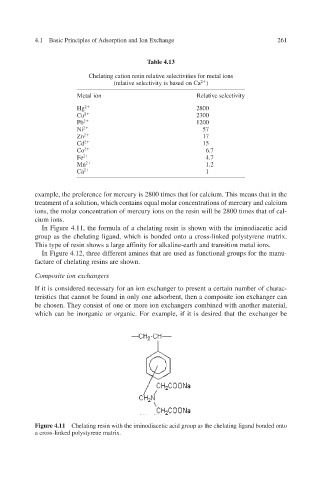
In Figure 4.11, the formula of a chelating resin is shown with the iminodiacetic acid
group as the chelating ligand, which is bonded onto a cross-linked polystyrene matrix.
This type of resin shows a large afinity for alkaline-earth and transition metal ions. f
In Figure 4.12, three different amines that are used as functional groups for the manu-
facture of chelating resins are sho wn.
xc er hang Composite ion es
If it is considered necessary for an ion exchanger to present a certain number of charac-
teristics that cannot be found in only one adsorbent, then a composite ion exchanger can
be chosen. They consist of one or more ion exchangers combined with another material,
which can be inorganic or organic. For e if it is desired that the exchanger be
xample,
Figure 4.11 Chelating resin with the iminodiacetic acid group as the chelating ligand bonded onto
a cross-linked polystyrene matrix.