Page 260 - Adsorption, Ion Exchange & Catalysis- 2007, Elsevier - Copy
P. 260
Else_AIEC-INGLE_cH004.qxd 7/1/2006 6:53 PM Page 256
256 4. Adsorption and Ion Exchange
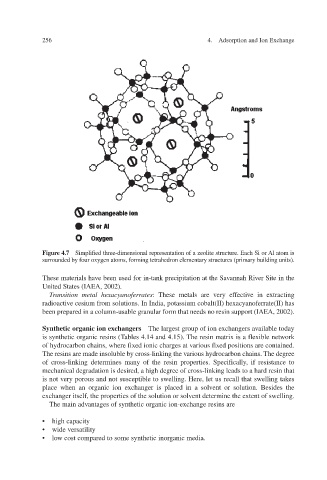
Figure 4.7 Simplified three-dimensional representation of a zeolite structure. Each Si or Al atom is
surrounded by four oxygen atoms, forming tetrahedron elementary structures (primary building units).
These materials have been used for in-tank precipitation at the Saer Site in the v v annah Ri
United States (IAEA, 2002).
v
fecti
Transition metal he xacyanoferr ates : These metals are very efe in e xtracting
radioactive cesium from solutions. In India, potassium cobalt(II) he xac yanoferrate(II) has
been prepared in a column-usable granular form that needs no resin support (IAEA, 2002).
Synthetic organic ion exchangers The largest group of ion exchangers a v ailable today
is synthetic organic resins (Tables 4.14 and 4.15). The resin matrix is a flexible netw ork
of hydrocarbon chains, where fges at v ix ed positions are contained. arious f ix ed ionic char
The resins are made insoluble by cross-linking the various hydrocarbon chains. The degree
,
ically
of cross-linking determines many of the resin properties. Specif if resistance to
mechanical degradation is desired, a high de gree of cross-linking leads to a hard resin that
is not very porous and not susceptible to swelling. Here, let us recall that swelling tak es
place when an organic ion exchanger is placed in a solvent or solution. Besides the
exchanger itself, the properties of the solution or solvent determine the e xtent of swelling.
The main advantages of synthetic organic ion-exchange resins are
• high capacity
• wide v ersatility
• low cost compared to some synthetic inorganic media.