Page 255 - Adsorption, Ion Exchange & Catalysis- 2007, Elsevier - Copy
P. 255
Else_AIEC-INGLE_cH004.qxd 7/1/2006 6:53 PM Page 251
4.1 Basic Principles of Adsorption and Ion Exchange 251
( 200 m 2 /g) and is highly porous. Peat moss is a relatie material and v xpensi v ely ine
is commercially sold at a price of 0.023 $/kg in the united States.
• Fly ash: Fly ash is an industrial solid wwer plants. Fly ash aste generated in thermal po
can be easily solidified after the heavy metals hae been adsorbed. v
• Iron (III) hydroxide waste: v w aste al from w Especially for heavy metals remoater, iron
,
(III) hydroxide waste and waste slurry from the fertilizer industry xanthate, rice husk,
carbon, and coconut shell hae been studied and can be considered as alternati v es. v
In Tables 4.4–4.7, seeral important adsorbents data are tab v ulated.
Safety consider ations
Adsorption is generally an exothermic process leading to temperature rise. Although this
property is useful when storage of heat is desired, it is not the case in the adsorption of
ed,
v
VOCs. If certain hydrocarbons are in the carbon or metals on the carbon may cat-
olv
alyze the oxidation of these pollutants when the adsorbent is hot, resulting in bed f ires that
deteriorate the adsorbent by either altering the its pore size or conerting part of it to ash. v
Cooling of the bed or humidification of the air can be employed to aoid the outburst of v
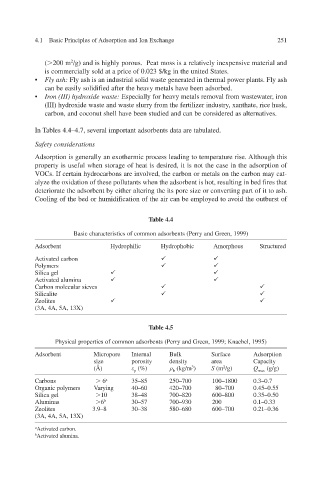
Table 4.4
Basic characteristics of common adsorbents (Perry and Green, 1999)
Adsorbent Hydrophilic Hydrophobic Amorphous Structured
Activated carbon
Polymers
Silica gel
Activated alumina
es v Carbon molecular sie
Silicalite
Zeolites
(3A, 4A, 5A, 13X)
Table 4.5
Physical properties of common adsorbents (Perry and Green, 1999; Knaebel, 1995)
Adsorbent Micropore Internal Bulk Surface Adsorption
size porosity density area Capacity
(Å) (%) (kg/m 3 ) S (m 2 /g) Q max (g/g)
p
b
Carbons 6 a 35–85 250–700 100–1800 0.3–0.7
Organic polymers Varying 40–60 420–700 80–700 0.45–0.55
Silica gel 10 38–48 700–820 600–800 0.35–0.50
Aluminas 6 b 30–57 700–930 200 0.1–0.33
Zeolites 3.9–8 30–38 580–680 600–700 0.21–0.36
(3A, 4A, 5A, 13X)
a Activated carbon.
b Activated alumina.