Page 254 - Adsorption, Ion Exchange & Catalysis- 2007, Elsevier - Copy
P. 254
Else_AIEC-INGLE_cH004.qxd 7/1/2006 6:53 PM Page 250
250 4. Adsorption and Ion Exchange
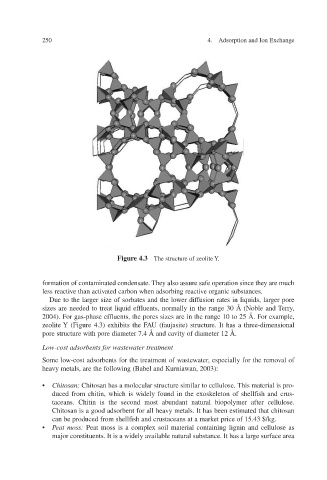
Figure 4.3 Y The structure of zeolite .
formation of contaminated condensate. They also assure safe operation since they are much
less reactive than activated carbon when adsorbing reactive organic substances.
Due to the larger size of sorbates and the lower diffusion rates in liquids, larger pore
fluents,
sizes are needed to treat liquid ef normally in the range 30 Å (Noble and T erry ,
fluents,
2004). For gas-phase ef the pores sizes are in the range 10 to 25 Å. For e xample,
A
U (f
zeolite Y (Figure 4.3) exhibits the Faujasite) structure. It has a three-dimensional
pore structure with pore diameter 7.4 Å and cavity of diameter 12 Å.
Low-cost adsorbents for wastewater tr eatment
w
aste
Some low-cost adsorbents for the treatment of w especially for the remoal of v
,
ater
heavy metals, are the following (Babel and K 2003): an, w urnia
• Chitosan: Chitosan has a molecular structure similar to cellulose. This material is pro-
xosk
duced from chitin, which is widely found in the eeleton of shellfish and crus-
taceans. Chitin is the second most abundant natural biopolymer after cellulose.
Chitosan is a good adsorbent for all heavy metals. It has been estimated that chitosan
can be produced from shellfish and crustaceans at a market price of 15.43 $/kg.
• Peat moss: Peat moss is a complex soil material containing lignin and cellulose as
major constituents. It is a widely available natural substance. It has a large surface area