Page 251 - Adsorption, Ion Exchange & Catalysis- 2007, Elsevier - Copy
P. 251
Else_AIEC-INGLE_cH004.qxd 7/1/2006 6:53 PM Page 247
4.1 Basic Principles of Adsorption and Ion Exchange 247
v
icienc
els,
y
, For optimum ef humidity le temperature, and pressure should be monitored
f
and controlled during the adsorption. The adsorption process of VOCs removal is exother-
mic in the most cases, which should be considered as a signif since , icant design parameter
there is a risk of fire in the remoal of high loads of organic compounds that exhibit high v
heats of adsorption.
Activated alumina
Activated alumina is amorphous or crystalline alumina, which has been partially or com-
pletly dehydrated and has a large surface area per unit mass. Actiated alumina is made v
from hydrated alumina, namelyAl , 2 O 3 . n H 2 O, where n by calcining to get 1~3, n closeto
0.5 (Knaebel, 1995). It is a white or tan-colored material of chalky appearance.
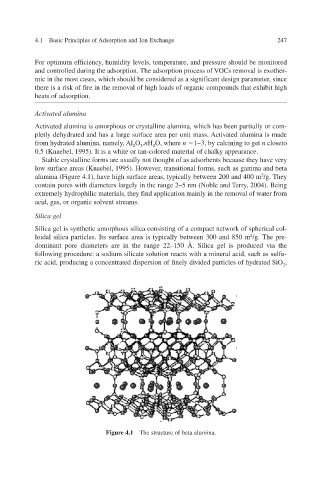
Stable crystalline forms are usually not thought of as adsorbents because they have very
low surface areas (Knaebel, 1995). Ho transitional forms, such as gamma and beta
,
er
we
v
alumina (Figure 4.1), hae high surface areas, typically between 200 and 400 m 2 /g. The y
v
contain pores with diameters largely in the range 2–5 nm (Noble and T2004). Being , erry
extremely hydrophilic materials, the ind application mainly in the removal of water from y f
acid, g or organic solvent streams. as,
Silica g el
Silica gel is synthetic amorphous silica consisting of a compact network of spherical col-
loidal silica particles. Its surface area is typically between 300 and 850 m 2 /g. The pre-
dominant pore diameters are in the range 22–150 Å. Silica gel is produced via the
following procedure: a sodium silicate solution reacts with a mineral acid, such as sulfu-
ric acid, producing a concentrated dispersion of finely divided particles of hydrated SiO 2 ,
Figure 4.1 The structure of beta alumina.