Page 253 - Adsorption, Ion Exchange & Catalysis- 2007, Elsevier - Copy
P. 253
Else_AIEC-INGLE_cH004.qxd 7/1/2006 6:53 PM Page 249
4.1 Basic Principles of Adsorption and Ion Exchange 249
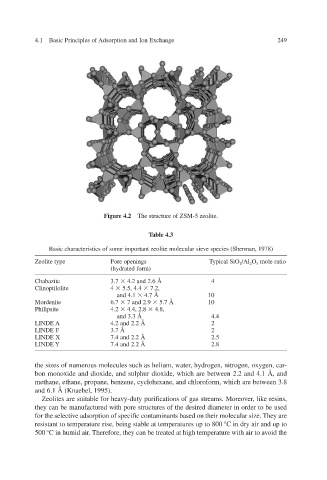
Figure 4.2 The structure of ZSM-5 zeolite.
Table 4.3
Basic characteristics of some important zeolite molecular siee species (Sherman, 1978) v
Zeolite type Pore openings Typical SiO /Al O mole ratio
2 2 3
(hydrated form)
Chabazite 3.7 4.2 and 2.6 Å 4
Clinoptilolite 4 5.5, 4.4 7.2,
and 4.1 4.7 Å 10
Mordenite 6.7 7 and 2.9 5.7 Å 10
Philipsite 4.2 4.4, 2.8 4.8,
and 3.3 Å 4.4
LINDE A 4.2 and 2.2 Å 2
LINDE F 3.7 Å 2
LINDE X 7.4 and 2.2 Å 2.5
LINDE Y 7.4 and 2.2 Å 2.8
ater
ydrogen,
the sizes of numerous molecules such as helium, w h nitrogen, oxygen, car-
,
bon monoxide and dioxide, and sulphur dioxide, which are between 2.2 and 4.1 Å, and
yclohe
methane, ethane, propane, benzene, c and chloroform, which are between 3.8
xane,
and 6.1 Å (Knaebel, 1995).
Zeolites are suitable for heaications of gas streams. Moreo like resins, vy-duty purif , er v
they can be manufactured with pore structures of the desired diameter in order to be used
for the selective adsorption of specific contaminants based on their molecular size. They are
resistant to temperature rise, being stable at temperatures up to 800 °C in dry air and up to
500°C in humid air. Therefore, the y can be treated at high temperature with air to avoid the