Page 37 - Adsorption, Ion Exchange & Catalysis- 2007, Elsevier - Copy
P. 37
Else_AIEC-INGLE_cH002.qxd 6/20/2006 11:31 AM Page 33
2.1 Def initions 33
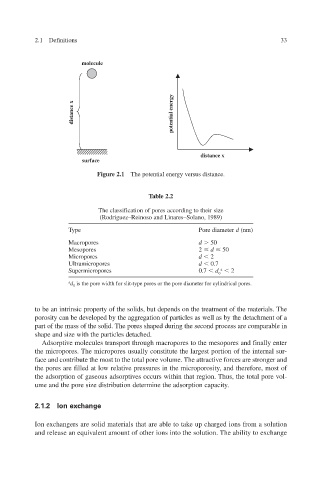
molecule
distance x
potential energy
distance x
surface
Figure 2.1 The potential energy versus distance.
Table 2.2
The classification of pores according to their size
(Rodriguez–Reinoso and Linares–Solano, 1989)
Type Pore diameter d (nm)
Macropores d 50
Mesopores 2 d 50
Micropores d 2
Ultramicropores d 0.7
Supermicropores 0.7 d o a 2
a d 0 is the pore width for slit-type pores or the pore diameter for cylindrical pores.
to be an intrinsic property of the solids, b The ut depends on the treatment of the materials.
porosity can be developed by the aggregation of particles as well as by the detachment of a
part of the mass of the solid. The pores shaped during the second process are comparable in
shape and size with the particles detached.
Adsorptive molecules transport through macropores to the mesopores and finally enter
the micropores. The micropores usually constitute the largest portion of the internal sur-
face and contribute the most to the total pore volume. The attractive forces are stronger and
the pores are filled at low relatie pressures in the microporosity and therefore, most of
v
,
the adsorption of gaseous adsorpties occurs within that reThus, the total pore v ol-
v
gion.
ume and the pore size distribution determine the adsorption capacity .
2.1.2 Ion exchange
Ion exchangers are solid materials that are able to take up charged ions from a solution
v
and release an equialent amount of other ions into the solution. The ability to exchange