Page 40 - Adsorption, Ion Exchange & Catalysis- 2007, Elsevier - Copy
P. 40
Else_AIEC-INGLE_cH002.qxd 6/20/2006 11:31 AM Page 36
36 2. Adsorption, Ion Exchange, and Catalysis
to acetaldehyde via the third reaction, dehydrated to ethylene via the fourth reaction, dehy-
drated to diethylether via the fifth reaction, and f inally decomposed to methane via the last
reaction. It is obvious that what could be misunderstood as a simple oxidation is really a
complex scheme of reactions. The presence of a catalyst may enhance one or more of these
reactions or even all of them by various degrees, leading to a different overall selectivity. Its
selection would be made on the basis of the desired products, and catalyst selectivity is the
key characteristic to practical applications.
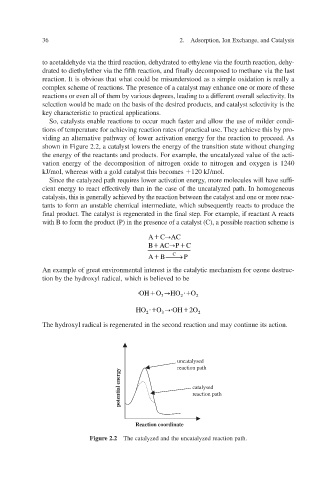
So, catalysts enable reactions to occur much faster and allow the use of milder condi-
tions of temperature for achieving reaction rates of practical use. They achieve this by pro-
v
v
viding an alternatie pathway of lower actiation energy for the reaction to proceed. As
shown in Figure 2.2, a catalyst lowers the energy of the transition state without changing
the energy of the reactants and products. For e the uncatalyzed value of the acti-
xample,
vation energy of the decomposition of nitrogen oxide to nitrogen and oxygen is 1240
kJ/mol, whereas with a gold catalyst this becomes 120 kJ/mol.
Since the catalyzed path requires lower activation energy, more molecules will have suffi-
fectively than in the case of the uncatalyzed path. In homogeneous cient energy to react ef
catalysis, this is generally achieved by the reaction between the catalyst and one or more reac-
tants to form an unstable chemical intermediate, which subsequently reacts to produce the
final product. The catalyst is regenerated in the final step. For example, if reactant A reacts
with B to form the product (P) in the presence of a catalyst (C), a possible reaction scheme is
AC A
C
BA P C
C
AB → C P
An example of great environmental interest is the catalytic mechanism for ozone destruc-
tion by the hydroxyl radical, which is belieed to be v
O HO 3 O 2 2
OH
O
HO O OH 2 2
3
2
The hydroxyl radical is regenerated in the second reaction and may continue its action.
uncatalysed
reaction path
catalysed
reaction path
potential energy
Reaction coordinate
Figure 2.2 The catalyzed and the uncatalyzed reaction path.