Page 277 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 277
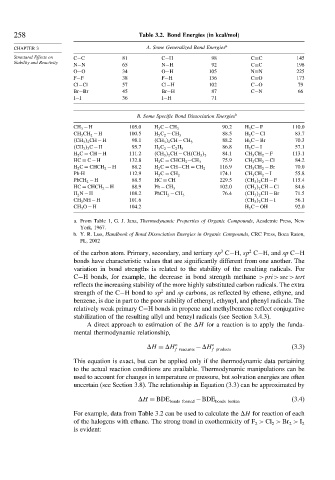
258 Table 3.2. Bond Energies (in kcal/mol)
CHAPTER 3 A. Some Generalized Bond Energies a
Structural Effects on C−C 81 C−H 98 C=C 145
Stability and Reactivity
N−N 65 N−H 92 C≡C 198
O−O 34 O−H 105 N≡N 225
F−F 38 F−H 136 C=O 173
Cl−Cl 57 Cl−H 102 C−O 79
Br−Br 45 Br−H 87 C−N 66
I−I 36 I−H 71
B. Some Specific Bond Dissociation Energies b
CH 3 −H 105 0 H 3 C−CH 3 90 2 H 3 C−F 110 0
CH 3 CH 2 −H 100 5 H 5 C 2 −CH 3 88 5 H 3 C−Cl 83 7
CH 3 2 CH−H 98 1 CH 3 2 CH−CH 3 88 2 H 3 C−Br 70 3
CH 3 3 C−H 95 7 H 5 C 2 −C 2 H 5 86 8 H 3 C−I 57 1
H 2 C = CH−H 111 2 CH 3 2 CH−CH CH 3 2 84 1 CH 3 CH 2 −F 113 1
HC ≡ C−H 132 8 H 2 C = CHCH 2 −CH 3 75 9 CH 3 CH 2 −Cl 84 2
H 2 C = CHCH 2 −H 88 2 H 2 C = CH−CH = CH 2 116 9 CH 3 CH 2 −Br 70 0
Ph-H 112 9 H 2 C = CH 2 174 1 CH 3 CH 2 −I 55 8
PhCH 2 −H 88 5 HC ≡ CH 229 5 CH 3 2 CH−F 115 4
HC ≡ CHCH 2 −H 88 9 Ph −CH 3 102 0 CH 3 2 CH−Cl 84 6
H 2 N −H 108 2 PhCH 2 −CH 3 76 4 CH 3 2 CH−Br 71 5
CH 3 NH−H 101 6 CH 3 2 CH−I 56 1
CH 3 O−H 104 2 H 3 C−OH 92 0
a. From Table 1, G. J. Janz, Thermodynamic Properties of Organic Compounds, Academic Press, New
York, 1967.
b. Y. R. Luo, Handbook of Bond Dissociation Energies in Organic Compounds, CRC Press, Boca Raton,
FL, 2002
3
2
of the carbon atom. Primary, secondary, and tertiary sp C−H, sp C−H, and sp C−H
bonds have characteristic values that are significantly different from one another. The
variation in bond strengths is related to the stability of the resulting radicals. For
C−H bonds, for example, the decrease in bond strength methane >pri>sec>tert
reflects the increasing stability of the more highly substituted carbon radicals. The extra
2
strength of the C−H bond to sp and sp carbons, as reflected by ethene, ethyne, and
benzene, is due in part to the poor stability of ethenyl, ethynyl, and phenyl radicals. The
relatively weak primary C−H bonds in propene and methylbenzene reflect conjugative
stabilization of the resulting allyl and benzyl radicals (see Section 3.4.3).
A direct approach to estimation of the H for a reaction is to apply the funda-
mental thermodynamic relationship,
H = H o − H o (3.3)
f reactants f products
This equation is exact, but can be applied only if the thermodynamic data pertaining
to the actual reaction conditions are available. Thermodynamic manipulations can be
used to account for changes in temperature or pressure, but solvation energies are often
uncertain (see Section 3.8). The relationship in Equation (3.3) can be approximated by
H = BDE bonds formed −BDE bonds broken (3.4)
For example, data from Table 3.2 can be used to calculate the H for reaction of each
of the halogens with ethane. The strong trend in exothermicity of F > Cl > Br > I 2
2
2
2
is evident: