Page 279 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 279
260 The original Pauling equation was reexamined recently by Zavitsas and co-
6
workers. The equation was shown to give quite good agreement with thermochemical
CHAPTER 3 data. Furthermore, it permitted assignment of electronegativity and stabilization energy
Structural Effects on to important radicals. The stabilization energy SE is assigned as
Stability and Reactivity
SE = 1/2 BDE CH3−CH3 −BDE X−X (3.8)
Some values are given in Table 3.3. For future reference, note the order of radical
stabilization: alkyl > alkenyl > alkynyl and allyl > benzyl > tertiary > secondary >
primary. In Section 3.4.3, we discuss the structural basis of these relationships.
Another idea underlying the nature of bond formation is the concept of electroneg-
8
7
ativity equalization or electronegativity equilibration (see Section 1.1.4). This
concept states that electron density flows from the less electronegative partner in
a bond (making it more positive and therefore more electronegative) to the more
electronegative atom (making it more negative and therefore less electronegative) until
both atoms have the same effective electronegativity. At that point, there is no net
attractive force on the electrons in the bond. This intuitively compelling idea has a
theoretical foundation in DFT, which states that the chemical potential
is uniform
throughout a molecule. It is observed that the apparent bond strength for several series
9
of compounds increases in the order CH − X < pri − X < sec − X tert − X. The
3
differences increase with the electronegativity of the substituent X. These electroneg-
ativity relationships lead to some qualitative trends. For alkyl groups with electroneg-
ative substituents, such as halogens, oxygen, or nitrogen, the trend is tert > sec > pri >
CH . On the other hand, for organometallics, alanes, and boranes, the order is reversed.
3
Compounds that can readily interconvert can isomerize in response to these stability
relationships. 10
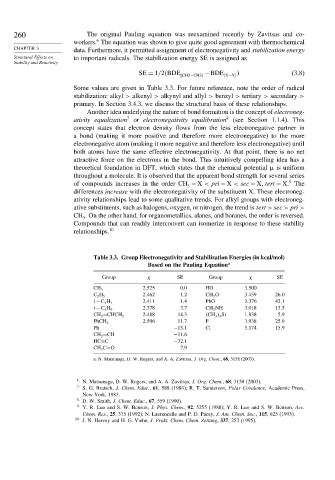
Table 3.3. Group Electronegativity and Stabilization Energies (in kcal/mol)
Based on the Pauling Equation a
Group SE Group SE
2 525 0.0 HO 3 500
CH 3
2 462 1.2 CH 3 O 3 439 26 0
C 2 H 5
2 411 1.4 PhO 3 376 43 1
i−C 3 H 7
2 378 3.7 CH 3 NH 3 018 13 5
t −C 4 H 9
2 488 14.3 CH 3 3 Si 1 838 5 9
CH 2 =CHCH 2
2 506 11.7 F 3 938 25 9
PhCH 2
Ph −13 1 Cl 3 174 15 9
CH 2 =CH −11 6
HC≡C −32 1
CH 3 C=O 7.9
a. N. Matsunaga, D. W. Rogers, and A. A. Zavitsas, J. Org. Chem., 68, 3158 (2003).
6
N. Matsunaga, D. W. Rogers, and A. A. Zavitsas, J. Org. Chem., 68, 3158 (2003).
7 S. G. Bratsch, J. Chem. Educ., 61, 588 (1984); R. T. Sanderson, Polar Covalence, Academic Press,
New York, 1983.
8
D. W. Smith, J. Chem. Educ., 67, 559 (1990).
9 Y. R. Luo and S. W. Benson, J. Phys. Chem., 92, 5255 (1988); Y. R. Luo and S. W. Benson, Acc.
Chem. Res., 25, 375 (1992); N. Laurencelle and P. D. Pacey, J. Am. Chem. Soc., 115, 625 (1993).
10
J. N. Harvey and H. G. Viehe, J. Prakt. Chem. Chem. Zeitung, 337, 253 (1995).