Page 396 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 396
b. Calculate H for the air oxidation of benzaldehyde to benzoic acid, given 377
that the H of benzaldehyde and benzoic acid are −8 8 and −70 1kcal/mol, PROBLEMS
f
respectively.
c. Using the appropriate heats of formation from Table 3.1, calculate the heat of
hydrogenation H H2 for 2-methyl-1-pentene.
3.2. Addition of methylmagnesium bromide to 2-methylcyclohexanone, followed by
iodine-catalyzed dehydration of the resulting alcohol gave three alkenes in the
ratio A:B:C = 3:31:66. Each alkene gave a mixture of cis- and trans-1,2-
dimethylcyclohexane upon catalytic hydrogenation. When the alkene mixture
was heated with a small amount of sulfuric acid, the ratio of A:B:C changed to
0.0:15:85. Assign structures to A, B, and C.
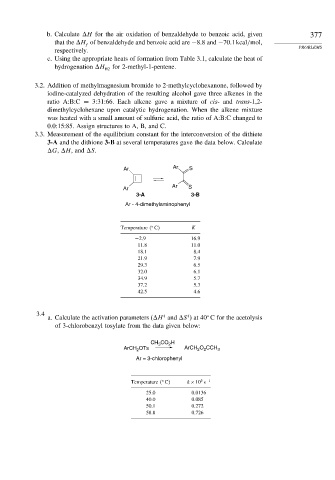
3.3. Measurement of the equilibrium constant for the interconversion of the dithiete
3-A and the dithione 3-B at several temperatures gave the data below. Calculate
G, H, and S.
Ar Ar S
Ar S
Ar
3-A 3-B
Ar - 4-dimethylaminophenyl
Temperature C K
−2 9 16.9
11.8 11.0
18.1 8.4
21.9 7.9
29.3 6.5
32.0 6.1
34.9 5.7
37.2 5.3
42.5 4.6
3.4
‡
‡
a. Calculate the activation parameters ( H and S )at40 C for the acetolysis
of 3-chlorobenzyl tosylate from the data given below:
CH CO H
3
2
ArCH OTs ArCH 2 O 2 CCH 3
2
Ar = 3-chlorophenyl
5 −1
Temperature C k×10 s
25.0 0.0136
40.0 0.085
50.1 0.272
58.8 0.726