Page 270 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 270
242 Scheme 3.3. (Continued)
CHAPTER 3
a. J. F. W. McOmie and D. E. West, Org. Synth., V, 412 (1973).
Functional Group b. P. A. Grieco, K. Hiroi, J. J. Reap, and J. A. Noguez, J. Org. Chem., 40, 1450 (1975).
Interconversion c. T. E. Jacks, D. T. Belmont, C. A. Briggs, N. M. Horne, G. D. Kanter, G. L. Karrick,
by Substitution, J. J. Krikke, R. J. McCabe, J. G. Mustakis, T. N. Nanninga, G. S. Risendorph, R. E. Seamans,
Including Protection and R. Skeean, D. D. Winkle, and T. M. Zennie, Org. Proc. Res. Dev., 8, 201 (2004).
Deprotection d. M. E. Jung and M. A. Lyster, Org. Synth., 59, 35 (1980).
e. T. Morita, Y. Okamoto, and H. Sakurai, J. Chem. Soc., Chem. Commun., 874 (1978).
f. E. H. Vickery, L. F. Pahler, and E. J. Eisenbraun, J. Org. Chem., 44, 4444 (1979).
g. K. Fuji, K. Ichikawa, M. Node, and E. Fujita, J. Org. Chem., 44, 1661 (1979).
h. M. Nobe, H. Hori, and E. Fujita, J. Chem. Soc. Perkin Trans., 1, 2237 (1976).
i. A. B. Smith, III, N. J. Liverton, N. J. Hrib, H. Sivaramakrishnan, and K. Winzenberg, J. Am.
Chem. Soc., 108, 3040 (1986).
j. Y. Guidon, M. Therien, Y. Girard, and C. Yoakim, J. Org. Chem., 52, 1680 (1987).
k. B. Ganem and V. R. Small, Jr., J. Org. Chem., 39, 3728 (1974).
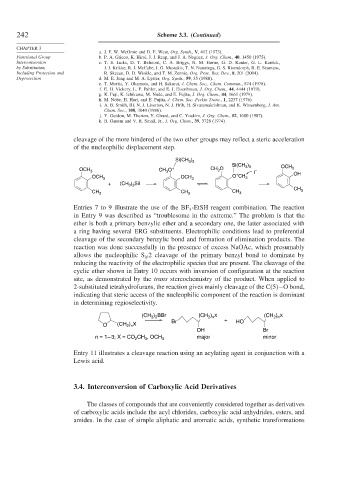
cleavage of the more hindered of the two ether groups may reflect a steric acceleration
of the nucleophilic displacement step.
Si(CH )
3 3
Si(CH ) OCH
3 3
OCH 3 CH O + CH O I – 3
3
3
+
OCH 3 OCH 3 O CH 3 OH
+ (CH ) SiI
3 3
CH
CH 3 CH 3 CH 3 3
Entries 7 to 9 illustrate the use of the BF -EtSH reagent combination. The reaction
3
in Entry 9 was described as “troublesome in the extreme.” The problem is that the
ether is both a primary benzylic ether and a secondary one, the latter associated with
a ring having several ERG substituents. Electrophilic conditions lead to preferential
cleavage of the secondary benzylic bond and formation of elimination products. The
reaction was done successfully in the presence of excess NaOAc, which presumably
allows the nucleophilic S 2 cleavage of the primary benzyl bond to dominate by
N
reducing the reactivity of the electrophilic species that are present. The cleavage of the
cyclic ether shown in Entry 10 occurs with inversion of configuration at the reaction
site, as demonstrated by the trans stereochemistry of the product. When applied to
2-substituted tetrahydrofurans, the reaction gives mainly cleavage of the C(5)−O bond,
indicating that steric access of the nucleophilic component of the reaction is dominant
in determining regioselectivity.
) BBr
) X
) X
(CH 3 2 (CH 2 n (CH 2 n
Br + HO
O (CH ) X
2 n
OH Br
n = 1 – 3; X = CO CH , OCH 3 major minor
3
2
Entry 11 illustrates a cleavage reaction using an acylating agent in conjunction with a
Lewis acid.
3.4. Interconversion of Carboxylic Acid Derivatives
The classes of compounds that are conveniently considered together as derivatives
of carboxylic acids include the acyl chlorides, carboxylic acid anhydrides, esters, and
amides. In the case of simple aliphatic and aromatic acids, synthetic transformations