Page 273 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 273
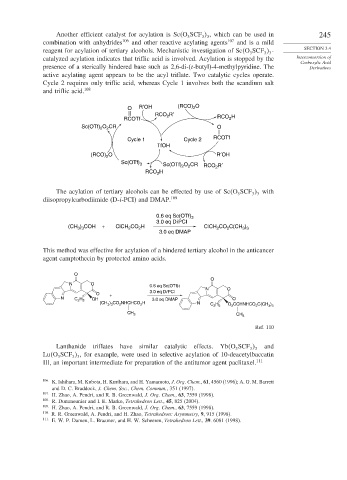
Another efficient catalyst for acylation is Sc O SCF , which can be used in 245
3 3
3
combination with anhydrides 106 and other reactive acylating agents 107 and is a mild
reagent for acylation of tertiary alcohols. Mechanistic investigation of Sc O SCF - SECTION 3.4
3
3 3
catalyzed acylation indicates that triflic acid is involved. Acylation is stopped by the Interconversion of
Carboxylic Acid
presence of a sterically hindered base such as 2,6-di-(t-butyl)-4-methylpyridine. The Derivatives
active acylating agent appears to be the acyl triflate. Two catalytic cycles operate.
Cycle 2 requires only triflic acid, whereas Cycle 1 involves both the scandium salt
and triflic acid. 108
O R′OH (RCO) 2 O
RCO R′
RCOTf 2 RCO 2 H
Sc(OTf) O CR O
2
2
Cycle 1 Cycle 2 RCOTf
TfOH
(RCO) O R′OH
2
Sc(OTf) O CR RCO R′
Sc(OTf) 3
2
2
2
RCO H
2
The acylation of tertiary alcohols can be effected by use of Sc O SCF with
3 3 3
diisopropylcarbodiimide (D-i-PCI) and DMAP. 109
0.6 eq Sc(OTf) 3
3.0 eq Di PCI
(CH ) COH + ClCH CO H ClCH CO C(CH )
2
2
3 3
3 3
2
2
3.0 eq DMAP
This method was effective for acylation of a hindered tertiary alcohol in the anticancer
agent camptothecin by protected amino acids.
O
O
N O
0.6 eq Sc(OTf)3
N O
3.0 eq Di PCI
O +
N H OH 3.0 eq DMAP O
C 2 5
(CH ) CO NHCHCO H N
3 3 2 2 C H O CCHNHCO C(CH )
2 5 2 2 3 3
CH
3 CH
3
Ref. 110
Lanthanide triflates have similar catalytic effects. Yb O SCF and
3 3
3
Lu O SCF , for example, were used in selective acylation of 10-deacetylbaccatin
3
3 3
III, an important intermediate for preparation of the antitumor agent paclitaxel. 111
106
K. Ishihara, M. Kubota, H. Kurihara, and H. Yamamoto, J. Org. Chem., 61, 4560 (1996); A. G. M. Barrett
and D. C. Braddock, J. Chem. Soc., Chem. Commun., 351 (1997).
107 H. Zhao, A. Pendri, and R. B. Greenwald, J. Org. Chem., 63, 7559 (1998).
108
R. Dummeunier and I. E. Marko, Tetrahedron Lett., 45, 825 (2004).
109
H. Zhao, A. Pendri, and R. B. Greenwald, J. Org. Chem., 63, 7559 (1998).
110 R. R. Greenwald, A. Pendri, and H. Zhao, Tetrahedron: Asymmetry, 9, 915 (1998).
111
E. W. P. Damen, L. Braamer, and H. W. Scheeren, Tetrahedron Lett., 39. 6081 (1998).