Page 277 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 277
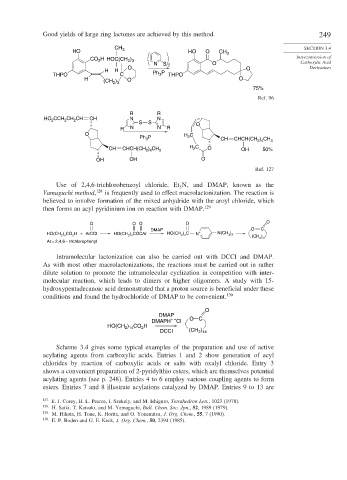
Good yields of large ring lactones are achieved by this method. 249
CH 3 SECTION 3.4
HO HO O CH 3
CO H HOC(CH ) Interconversion of
2
2 3
N S 2 O Carboxylic Acid
O O Derivatives
H H
THPO C Ph 3 P THPO
H O O
)
(CH 2 3
75%
Ref. 96
R R
CCH CH CH CH N N
HO 2 2 2
S S O
R N N R
O H C
Ph P 2 CH CHCH(CH ) CH 3
3
2 4
CH CHCH(CH ) CH 3 H C O OH 50%
2
2 4
OH OH O
Ref. 127
Use of 2,4,6-trichlorobenzoyl chloride, Et N, and DMAP, known as the
3
Yamaguchi method, 128 is frequently used to effect macrolactonization. The reaction is
believed to involve formation of the mixed anhydride with the aroyl chloride, which
then forms an acyl pyridinium ion on reaction with DMAP. 129
O
O O O O
DMAP O C
HO(CH ) CO H+ ArCCl HO(CH 2 n ) COCAr HO(CH ) C N + N(CH )
3 2
2 n
2
2 n
(CH )
2 n
Ar = 2,4,6 – trichlorophenyl
Intramolecular lactonization can also be carried out with DCCI and DMAP.
As with most other macrolactonizations, the reactions must be carried out in rather
dilute solution to promote the intramolecular cyclization in competition with inter-
molecular reaction, which leads to dimers or higher oligomers. A study with 15-
hydroxypentadecanoic acid demonstrated that a proton source is beneficial under these
conditions and found the hydrochloride of DMAP to be convenient. 130
O
DMAP
DMAPH + – Cl OC
HO(CH ) CO H
2
2 14
DCCI (CH )
2 14
Scheme 3.4 gives some typical examples of the preparation and use of active
acylating agents from carboxylic acids. Entries 1 and 2 show generation of acyl
chlorides by reaction of carboxylic acids or salts with oxalyl chloride. Entry 3
shows a convenient preparation of 2-pyridylthio esters, which are themselves potential
acylating agents (see p. 248). Entries 4 to 6 employ various coupling agents to form
esters. Entries 7 and 8 illustrate acylations catalyzed by DMAP. Entries 9 to 13 are
127
E. J. Corey, H. L. Pearce, I. Szekely, and M. Ishiguro, Tetrahedron Lett., 1023 (1978).
128
H. Saiki, T. Katsuki, and M. Yamaguchi, Bull. Chem. Soc. Jpn., 52, 1989 (1979).
129 M. Hikota, H. Tone, K. Horita, and O. Yonemitsu, J. Org. Chem., 55, 7 (1990).
130
E. P. Boden and G. E. Keck, J. Org. Chem., 50, 2394 (1985).