Page 282 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 282
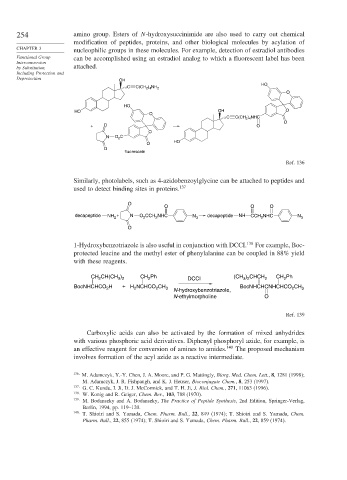
254 amino group. Esters of N-hydroxysuccinimide are also used to carry out chemical
modification of peptides, proteins, and other biological molecules by acylation of
CHAPTER 3
nucleophilic groups in these molecules. For example, detection of estradiol antibodies
Functional Group can be accomplished using an estradiol analog to which a fluorescent label has been
Interconversion
by Substitution, attached.
Including Protection and
Deprotection OH
HO
C C(CH 2 ) 4 NH 2
O
HO
HO OH O
O
C C(CH 2 ) 4 NHC
O
+ O O
O
N O 2 C
HO
O
O
fluorescein
Ref. 136
Similarly, photolabels, such as 4-azidobenzoylglycine can be attached to peptides and
used to detect binding sites in proteins. 137
O
O O O
decapeptide NH + N O 2 CCH NHC N 3 decapeptide NH CCH NHC N 3
2
2
2
O
1-Hydroxybenzotriazole is also useful in conjunction with DCCI. 138 For example, Boc-
protected leucine and the methyl ester of phenylalanine can be coupled in 88% yield
with these reagents.
CH CH(CH ) CH Ph DCCI (CH ) CHCH 2 CH Ph
2
3 2
2
3 2
2
BocNHCHCO H + H NCHCO CH 3 BocNHCHCNHCHCO 2 CH 3
2
2
2
N-hydroxybenzotriazole,
N-ethylmorpholine O
Ref. 139
Carboxylic acids can also be activated by the formation of mixed anhydrides
with various phosphoric acid derivatives. Diphenyl phosphoryl azide, for example, is
an effective reagent for conversion of amines to amides. 140 The proposed mechanism
involves formation of the acyl azide as a reactive intermediate.
136 M. Adamczyk, Y.-Y. Chen, J. A. Moore, and P. G. Mattingly, Biorg. Med. Chem. Lett., 8, 1281 (1998);
M. Adamczyk, J. R. Fishpaugh, and K. J. Heuser, Bioconjugate Chem., 8, 253 (1997).
137 G. C. Kundu, I. Ji, D. J. McCormick, and T. H. Ji, J. Biol. Chem., 271, 11063 (1996).
138
W. Konig and R. Geiger, Chem. Ber., 103, 788 (1970).
139 M. Bodanszky and A. Bodanszky, The Practice of Peptide Synthesis, 2nd Edition, Springer-Verlag,
Berlin, 1994, pp. 119–120.
140
T. Shioiri and S. Yamada, Chem. Pharm. Bull., 22, 849 (1974); T. Shioiri and S. Yamada, Chem.
Pharm. Bull., 22, 855 (1974); T. Shioiri and S. Yamada, Chem. Pharm. Bull., 22, 859 (1974).