Page 287 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 287
reagents. The acidic proton of a hydroxy group will destroy one equivalent of a strongly 259
basic organometallic reagent and possibly adversely affect the reaction in other ways.
In some cases, protection of the hydroxy group also improves the solubility of alcohols SECTION 3.5
in nonpolar solvents. The choice of the most appropriate group is largely dictated by Installation and Removal
of Protective Groups
the conditions that can be tolerated in subsequent removal of the protecting group.
The tetrahydropyranyl ether (THP) is applicable when mildly acidic hydrolysis is an
appropriate method for deprotection. 150 The THP group, like other acetals and ketals,
is inert to basic and nucleophilic reagents and is unchanged under such conditions
as hydride reduction, organometallic reactions, or base-catalyzed reactions in aqueous
solution. It also protects the hydroxy group against oxidation. The THP group is
introduced by an acid-catalyzed addition of the alcohol to the vinyl ether moiety in
dihydropyran. p-Toluenesulfonic acid or its pyridinium salt are frequently used as the
catalyst, 151 although other catalysts are advantageous in special cases.
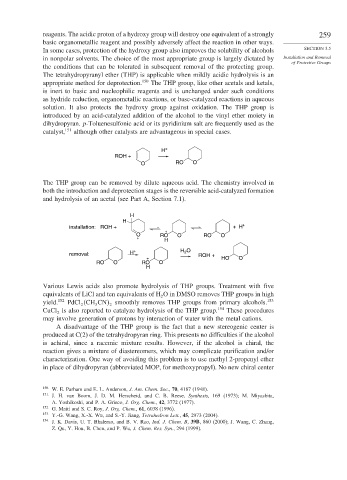
H +
ROH +
O RO O
The THP group can be removed by dilute aqueous acid. The chemistry involved in
both the introduction and deprotection stages is the reversible acid-catalyzed formation
and hydrolysis of an acetal (see Part A, Section 7.1).
H
H
installation: ROH + + H +
+
O RO O RO O
+
H
2
removal: H + H O ROH +
+ HO O
RO O RO O
H
Various Lewis acids also promote hydrolysis of THP groups. Treatment with five
equivalents of LiCl and ten equivalents of H O in DMSO removes THP groups in high
2
yield. 152 PdCl CH CN smoothly removes THP groups from primary alcohols. 153
3
2
2
CuCl is also reported to catalyze hydrolysis of the THP group. 154 These procedures
2
may involve generation of protons by interaction of water with the metal cations.
A disadvantage of the THP group is the fact that a new stereogenic center is
produced at C(2) of the tetrahydropyran ring. This presents no difficulties if the alcohol
is achiral, since a racemic mixture results. However, if the alcohol is chiral, the
reaction gives a mixture of diastereomers, which may complicate purification and/or
characterization. One way of avoiding this problem is to use methyl 2-propenyl ether
in place of dihydropyran (abbreviated MOP, for methoxypropyl). No new chiral center
150
W. E. Parham and E. L. Anderson, J. Am. Chem. Soc., 70, 4187 (1948).
151
J. H. van Boom, J. D. M. Herscheid, and C. B. Reese, Synthesis, 169 (1973); M. Miyashita,
A. Yoshikoshi, and P. A. Grieco, J. Org. Chem., 42, 3772 (1977).
152 G. Maiti and S. C. Roy, J. Org. Chem., 61, 6038 (1996).
153 Y.-G. Wang, X.-X. Wu, and S.-Y. Jiang, Tetrahedron Lett., 45, 2973 (2004).
154
J. K. Davis, U. T. Bhalerao, and B. V. Rao, Ind. J. Chem. B, 39B, 860 (2000); J. Wang, C. Zhang,
Z. Qu, Y. Hou, B. Chen, and P. Wu, J. Chem. Res. Syn., 294 (1999).