Page 285 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 285
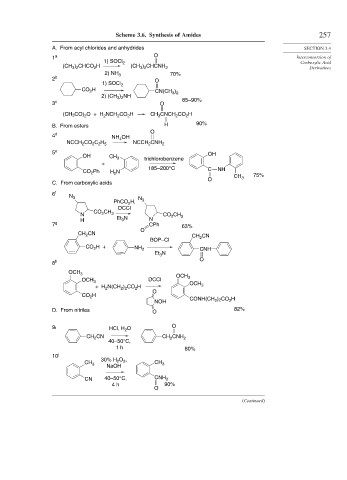
Scheme 3.6. Synthesis of Amides 257
A. From acyl chlorides and anhydrides SECTION 3.4
1 a O Interconversion of
1) SOCl 2 Carboxylic Acid
(CH ) CHCO H (CH ) CHCNH 2 Derivatives
3 2
2
3 2
2) NH 3 70%
2 b O
1) SOCl 2
H
CO 2 )
CN(CH 3 2
2) (CH ) NH
3 2
3 c O 85–90%
(CH 3CO) O + H NCH CO H CH CNCH CO H
3
2
2
2
2
2
2
B. From esters H 90%
O
4 d NH OH
4
CO C H CNH
NCCH 2 2 2 5 NCCH 2 2
5 e OH
OH CH 3 trichlorobenzene
+
185–200°C C NH
CO 2 Ph H N CH 75%
2
O 3
C. From carboxylic acids
6 f N 3
PhCO H, N 3
2
DCCI
CO CH
N 2 3 CO CH 3
2
3
H Et N N
7 g CPh 63%
O
CH 2 CN
CH CN
BOP–Cl 2
CO 2 H+ NH 2 CNH
Et N
3
O
8 h
OCH 3 OCH
OCH 3 DCCI 3
+H N(CH ) CO H OCH 3
2
2
2 2
O
CO H
2
2
NOH CONH(CH ) 2 CO H
2
D. From nitriles O 82%
9i HCl, H 2 O O
CN CH CNH
CH 2 2 2
40–50°C,
1 h 80%
10 j
2
CH 3 30% H 2 O , CH 3
NaOH
CN 40–50°C, CNH 2
4 h 90%
O
(Continued)