Page 278 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 278
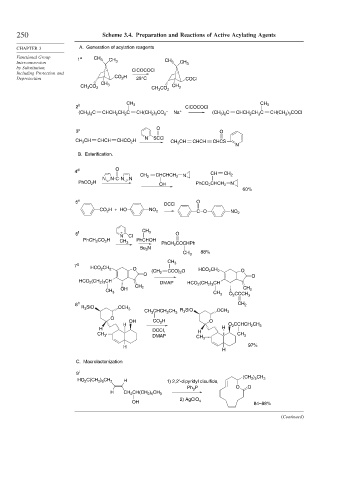
250 Scheme 3.4. Preparation and Reactions of Active Acylating Agents
CHAPTER 3 A. Generation of acylation reagents
Functional Group 1 a CH CH
Interconversion 3 3 CH 3 CH 3
by Substitution, ClCOCOCl
Including Protection and
Deprotection CO 2 H 25°C COCl
CH 3
CH 3 CO 2 CH 3
CH 3 CO 2
2 b CH 3 ClCOCOCl CH 3
–
(CH 3 ) 2 C CHCH 2 CH 2 C CH(CH 2 ) 3 CO 2 Na + (CH 3 ) 2 C CHCH 2 CH 2 C CH(CH 2 ) 3 COCl
3 c O O
N SCCl
CH 3 CH CHCH CHCO 2 H CH 3 CH CHCH CHCS
N
B. Esterification.
4 d O
CH 2 CHCHCH 2 N CH CH 2
N N C N N
PhCO 2 H N
OH PhCO 2 CHCH 2
60%
5 e O
DCCI
CO 2 H + HO NO 2 CO NO 2
6 f + CH 3 O
N Cl
PhCH 2 CO 2 H PhCHOH
PhCH 2 COCHPh
CH 3
Bu 3 N
88%
CH 3
7 g CH 3
HCO 2 CH 2 O (CH 2 CCO) 2 O HCO 2 CH 2 O
O O
HCO 2 (CH 2 ) 3 CH DMAP HCO 2 (CH 2 ) 3 CH
CH 2
OH CH 2
CH 3
CH 3
O 2 CCCH 3
8 h CH 2
R 3 SiO OCH 3
R 3 SiO
CH 3 CHCH 2 CH 3 OCH 3
O
OH CO 2 H O
H O 2 CCHCH 2 CH 3
H DCCI, H H
DMAP CH 3
CH 3 CH 3
97%
H
H
C. Macrolactonization
9 i
(CH 2 ) 5 CH 3
H
HO 2 C(CH 2 ) 6 CH 2 1) 2,2′-dipyridyl disulfide,
Ph 3 P O O
H CH 2 CH(CH 2 ) 5 CH 3
OH 2) AgClO 4
84–88%
(Continued)