Page 276 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 276
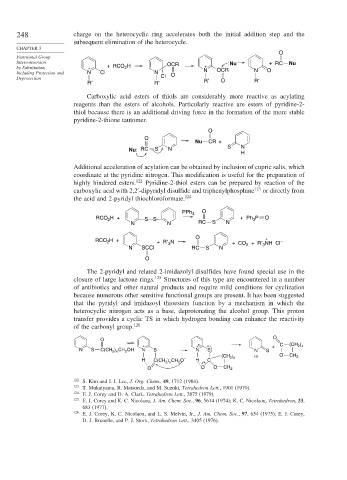
248 charge on the heterocyclic ring accelerates both the initial addition step and the
subsequent elimination of the heterocycle.
CHAPTER 3
O
Functional Group
Interconversion OCR :Nu + RC Nu
+
by Substitution, + + RCO 2 H N OCR N O
Including Protection and N Cl N O
Deprotection Cl R′ R′
R′ R′ O
Carboxylic acid esters of thiols are considerably more reactive as acylating
reagents than the esters of alcohols. Particularly reactive are esters of pyridine-2-
thiol because there is an additional driving force in the formation of the more stable
pyridine-2-thione tautomer.
O
O
Nu CR +
Nu: RC S N S N
H
Additional acceleration of acylation can be obtained by inclusion of cupric salts, which
coordinate at the pyridine nitrogen. This modification is useful for the preparation of
highly hindered esters. 122 Pyridine-2-thiol esters can be prepared by reaction of the
carboxylic acid with 2,2 -dipyridyl disulfide and triphenylphosphine 123 or directly from
the acid and 2-pyridyl thiochloroformate. 124
O
PPh 3
RCO H + SS + Ph P O
3
2
N N RC S N
O
H + +
RCO 2 + R′ N + CO 2 + R′ NH Cl –
3
N SCCl RC S N 3
O
The 2-pyridyl and related 2-imidazolyl disulfides have found special use in the
closure of large lactone rings. 125 Structures of this type are encountered in a number
of antibiotics and other natural products and require mild conditions for cyclization
because numerous other sensitive functional groups are present. It has been suggested
that the pyridyl and imidazoyl thioesters function by a mechanism in which the
heterocyclic nitrogen acts as a base, deprotonating the alcohol group. This proton
transfer provides a cyclic TS in which hydrogen bonding can enhance the reactivity
of the carbonyl group. 126
O
O
C
2
+ + + (CH ) x
N S C(CH ) CH OH N S N S N S
2
2 x
2
(CH ) x H O CH 2
H C(CH ) CH O – H – C
2 x
2
O O O CH 2
122 S. Kim and J. I. Lee, J. Org. Chem., 49, 1712 (1984).
123
T. Mukaiyama, R. Matsueda, and M. Suzuki, Tetrahedron Lett., 1901 (1970).
124 E. J. Corey and D. A. Clark, Tetrahedron Lett., 2875 (1979).
125 E. J. Corey and K. C. Nicolaou, J. Am. Chem. Soc., 96, 5614 (1974); K. C. Nicolaou, Tetrahedron, 33,
683 (1977).
126
E. J. Corey, K. C. Nicolaou, and L. S. Melvin, Jr., J. Am. Chem. Soc., 97, 654 (1975); E. J. Corey,
D. J. Brunelle, and P. J. Stork, Tetrahedron Lett., 3405 (1976).