Page 30 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 30
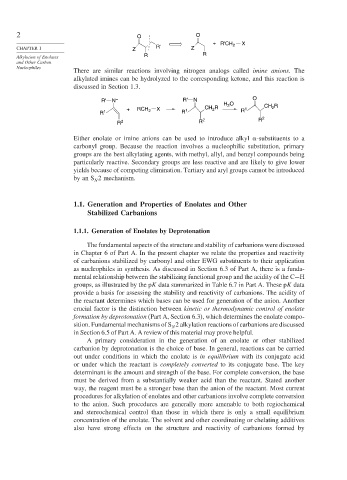
2 O O
+ R'CH 2 X
CHAPTER 1 Z R' Z
Alkylation of Enolates R R
and Other Carbon
Nucleophiles
There are similar reactions involving nitrogen analogs called imine anions. The
alkylated imines can be hydrolyzed to the corresponding ketone, and this reaction is
discussed in Section 1.3.
R' N – R' N O
H O CH R
2
+ RCH 2 X 1 CH 2 R R 1 2
R 1 R
R 2 R 2 R 2
Either enolate or imine anions can be used to introduce alkyl -substituents to a
carbonyl group. Because the reaction involves a nucleophilic substitution, primary
groups are the best alkylating agents, with methyl, allyl, and benzyl compounds being
particularly reactive. Secondary groups are less reactive and are likely to give lower
yields because of competing elimination. Tertiary and aryl groups cannot be introduced
by an S 2 mechanism.
N
1.1. Generation and Properties of Enolates and Other
Stabilized Carbanions
1.1.1. Generation of Enolates by Deprotonation
The fundamental aspects of the structure and stability of carbanions were discussed
in Chapter 6 of Part A. In the present chapter we relate the properties and reactivity
of carbanions stabilized by carbonyl and other EWG substituents to their application
as nucleophiles in synthesis. As discussed in Section 6.3 of Part A, there is a funda-
mental relationship between the stabilizing functional group and the acidity of the C−H
groups, as illustrated by the pK data summarized in Table 6.7 in Part A. These pK data
provide a basis for assessing the stability and reactivity of carbanions. The acidity of
the reactant determines which bases can be used for generation of the anion. Another
crucial factor is the distinction between kinetic or thermodynamic control of enolate
formation by deprotonation (Part A, Section 6.3), which determines the enolate compo-
sition. Fundamental mechanisms of S 2 alkylation reactions of carbanions are discussed
N
in Section 6.5 of Part A. A review of this material may prove helpful.
A primary consideration in the generation of an enolate or other stabilized
carbanion by deprotonation is the choice of base. In general, reactions can be carried
out under conditions in which the enolate is in equilibrium with its conjugate acid
or under which the reactant is completely converted to its conjugate base. The key
determinant is the amount and strength of the base. For complete conversion, the base
must be derived from a substantially weaker acid than the reactant. Stated another
way, the reagent must be a stronger base than the anion of the reactant. Most current
procedures for alkylation of enolates and other carbanions involve complete conversion
to the anion. Such procedures are generally more amenable to both regiochemical
and stereochemical control than those in which there is only a small equilibrium
concentration of the enolate. The solvent and other coordinating or chelating additives
also have strong effects on the structure and reactivity of carbanions formed by