Page 453 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 453
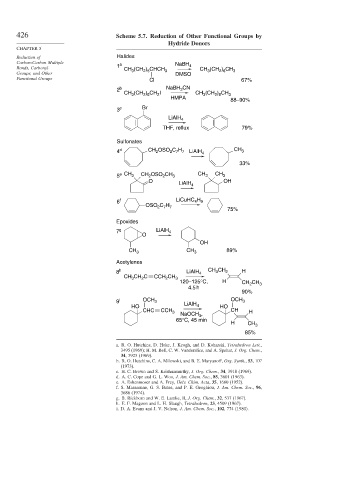
426 Scheme 5.7. Reduction of Other Functional Groups by
Hydride Donors
CHAPTER 5
Reduction of Halides
Carbon-Carbon Multiple a NaBH
Bonds, Carbonyl 1 (CH ) CHCH 4 CH (CH ) CH
CH 3 2 5 3 3 2 6 3
Groups, and Other DMSO
Functional Groups Cl 67%
2 b NaBH CN
3
3
2 8
2 8
CH 3 (CH ) CH I CH (CH ) CH 3
2
HMPA
88–90%
3 c Br
LiAlH 4
THF, reflux 79%
Sulfonates
4 d CH OSO C H 4 CH 3
2 7 7 LiAlH
2
33%
5 e CH 3 CH OSO CH 3 CH 3 CH 3
2
2
O OH
LiAlH 4
6 f LiCuHC H
4 9
OSO C H
2 7 7
75%
Epoxides
7 g LiAlH 4
O
OH
CH 3 CH 3 89%
Acetylenes
8 h LiAlH 4 CH CH 2 H
3
CH CH C CCH 2 CH 3
3
2
120–125°C, H CH CH 3
2
4.5 h
90%
9 i OCH 3 LiAlH OCH 3
HO 4 HO
CHC CCH 3 CH H
NaOCH ,
3
65°C, 45 min
H CH 3
85%
a. R. O. Hutchins, D. Hoke, J. Keogh, and D. Koharski, Tetrahedron Lett.,
3495 (1969); H. M. Bell, C. W. Vanderslice, and A. Spehar, J. Org. Chem.,
34, 3923 (1969).
b. R. O. Hutchins, C. A. Milewski, and B. E. Maryanoff, Org. Synth., 53, 107
(1973).
c. H. C. Brown and S. Krishnamurthy, J. Org. Chem., 34, 3918 (1969).
d. A. C. Cope and G. L. Woo, J. Am. Chem. Soc., 85, 3601 (1963).
e. A. Eshenmoser and A. Frey, Helv. Chim. Acta, 35, 1660 (1952).
f. S. Masamune, G. S. Bates, and P. E. Geoghiou, J. Am. Chem. Soc., 96,
3686 (1974).
g. B. Rickborn and W. E. Lamke, II, J. Org. Chem., 32, 537 (1967).
h. E. F. Magoon and L. H. Slaugh, Tetrahedron, 23, 4509 (1967).
i. D. A. Evans and J. V. Nelson, J. Am. Chem. Soc., 102, 774 (1980).