Page 459 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 459
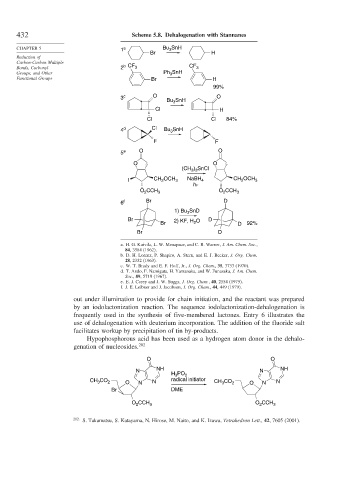
432 Scheme 5.8. Dehalogenation with Stannanes
CHAPTER 5 1 a Bu SnH
3
Br H
Reduction of
Carbon-Carbon Multiple
Bonds, Carbonyl 2 b CF 3 CF 3
Groups, and Other Ph SnH
3
Functional Groups Br H
99%
3 c O O
Bu SnH
3
Cl H
Cl Cl 84%
4 d Cl Bu SnH
3
F F
5 e O O
O O
(CH ) SnCl
3 3
I CH OCH 3 NaBH 4 CH OCH 3
2
2
hν
O CCH 3 O CCH 3
2
2
6 f Br D
1) Bu SnD
3
Br 2) KF, H O D
Br 2 D 92%
Br D
a. H. G. Kuivila, L. W. Menapace, and C. R. Warner, J. Am. Chem. Soc.,
84, 3584 (1962).
b. D. H. Lorenz, P. Shapiro, A. Stern, and E. J. Becker, J. Org. Chem.
28, 2332 (1963).
c. W. T. Brady and E. F. Hoff, Jr., J. Org. Chem., 35, 3733 (1970).
d. T. Ando, F. Namigata, H. Yamanaka, and W. Funasaka, J. Am. Chem.
Soc., 89, 5719 (1967).
e. E. J. Corey and J. W. Suggs, J. Org. Chem., 40, 2554 (1975).
f. J. E. Leibner and J. Jacobson, J. Org. Chem., 44, 449 (1979).
out under illumination to provide for chain initiation, and the reactant was prepared
by an iodolactonization reaction. The sequence iodolactonization-dehalogenation is
frequently used in the synthesis of five-membered lactones. Entry 6 illustrates the
use of dehalogenation with deuterium incorporation. The addition of the fluoride salt
facilitates workup by precipitation of tin by-products.
Hypophosphorous acid has been used as a hydrogen atom donor in the dehalo-
genation of nucleosides. 202
O O
N NH N NH
H PO 2
3
CO radical initiator
CH 3 2 O N N CH CO 2 O N N
3
Br DME
2
O 2 CCH 3 O CCH 3
202
S. Takamatsu, S. Katayama, N. Hirose, M. Naito, and K. Izawa, Tetrahedron Lett., 42, 7605 (2001).