Page 461 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 461
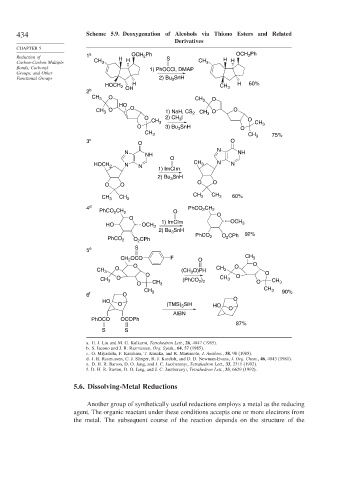
434 Scheme 5.9. Deoxygenation of Alcohols via Thiono Esters and Related
Derivatives
CHAPTER 5
2
Reduction of 1 a H OCH 2 Ph S OCH Ph
Carbon-Carbon Multiple CH 3 H CH 3 H H
Bonds, Carbonyl 1) PhOCCl, DMAP
Groups, and Other
Functional Groups 2) Bu SnH
3
HOCH 2 H CH 3 H 60%
2 b OH
CH 3 O CH 3 O
HO
CH O O 1) NaH, CS 2 CH O O
3
3
O 2) CH 3 I O
CH 3 CH
O 3) Bu SnH O 3
3
CH 3 CH 3 75%
3 c O O
N N NH
NH
O
N CH 3 N N
HOCH 2 N
1) ImCIm
2) Bu SnH
3
O O O O
CH 3 CH 3 CH 3 CH 3 60%
4 d PhCO 2 CH 2
PhCO CH 2 O
2
O O
HO OCH 3 1) ImCIm OCH 3
2) Bu 3 SnH 92%
2
PhCO 2 O 2 CPh PhCO 2 O CPh
5 e S
CH 2 OCO F O CH 3
O O O
CH 3 O (CH O)PH CH 3 O
3
CH 3 O O (PhCO 2 ) 2 CH 3 O CH
O CH 3 O 3
CH 3 CH 3 90%
6 f O
HO O
O (TMS) 3 SiH HO
O
AIBN
PhOCO OCOPh
87%
S S
a. H. J. Liu and M. G. Kulkarni, Tetrahedron Lett., 26, 4847 (1985).
b. S. Iacono and J. R. Rasmussen, Org. Synth., 64, 57 (1985).
c. O. Miyashita, F. Kasahara, T. Kusaka, and R. Marumoto, J. Antibiot., 38, 98 (1985).
d. J. R. Rasmussen, C. J. Slinger, R. J. Kordish, and D. D. Newman-Evans, J. Org. Chem., 46, 4843 (1981).
e. D. H. R. Barton, D. O. Jang, and J. C. Jaszberenyi, Tetrahedron Lett., 33, 2311 (1992).
f. D. H. R. Barton, D. O. Jang, and J. C. Jaszberenyi, Tetrahedron Lett., 33, 6629 (1992).
5.6. Dissolving-Metal Reductions
Another group of synthetically useful reductions employs a metal as the reducing
agent. The organic reactant under these conditions accepts one or more electrons from
the metal. The subsequent course of the reaction depends on the structure of the