Page 465 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 465
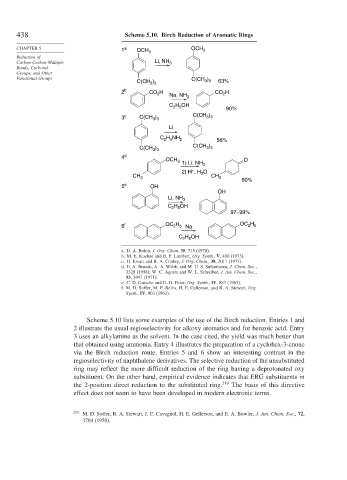
438 Scheme 5.10. Birch Reduction of Aromatic Rings
CHAPTER 5 1 a OCH 3 OCH 3
Reduction of
Carbon-Carbon Multiple Li, NH 3
Bonds, Carbonyl
Groups, and Other
Functional Groups C(CH )
C(CH ) 3 3 63%
3 3
2 b CO H Na, NH 3 CO H
2
2
C H OH
2 5
90%
3 3
3 c C(CH ) C(CH )
3 3
Li
C H NH 2 56%
2 5
C(CH )
C(CH ) 3 3
3 3
4 d
OCH 3 1) Li, NH 3 O
+
2) H , H O
CH 3 2 CH 3
80%
5 e OH
OH
Li, NH 3
H OH
C 2 5
97–99%
H
6 f OC 2 5 Na OC H
2 5
C H OH
2 5
a. D. A. Bolon, J. Org. Chem. 35, 715 (1970).
b. M. E. Kuehne and B. F. Lambert, Org. Synth., V, 400 (1973).
c. H. Kwart and R. A. Conley, J. Org. Chem., 38, 2011 (1973).
d. E. A. Braude, A. A. Webb, and M. U. S. Sultanbawa, J. Chem. Soc.,
3328 (1958); W. C. Agosta and W. L. Schreiber, J. Am. Chem. Soc.,
93, 3947 (1971).
e. C. D. Gutsche and H. H. Peter, Org. Synth., IV, 887 (1963).
f. M. D. Soffer, M. P. Bellis, H. E. Gellerson, and R. A. Stewart, Org.
Synth., IV, 903 (1963).
Scheme 5.10 lists some examples of the use of the Birch reduction. Entries 1 and
2 illustrate the usual regioselectivity for alkoxy aromatics and for benzoic acid. Entry
3 uses an alkylamine as the solvent. In the case cited, the yield was much better than
that obtained using ammonia. Entry 4 illustrates the preparation of a cyclohex-3-enone
via the Birch reduction route. Entries 5 and 6 show an interesting contrast in the
regioselectivity of naphthalene derivatives. The selective reduction of the unsubstituted
ring may reflect the more difficult reduction of the ring having a deprotonated oxy
substituent. On the other hand, empirical evidence indicates that ERG substituents in
the 2-position direct reduction to the substituted ring. 219 The basis of this directive
effect does not seem to have been developed in modern electronic terms.
219
M. D. Soffer, R. A. Stewart, J. C. Cavagnol, H. E. Gellerson, and E. A. Bowler, J. Am. Chem. Soc., 72,
3704 (1950).