Page 469 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 469
442 –
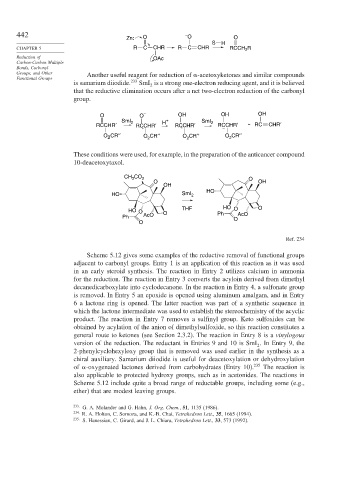
Zn: O O O
S H
CHAPTER 5 R C CHR R C CHR RCCH 2 R
Reduction of OAc
Carbon-Carbon Multiple
Bonds, Carbonyl
Groups, and Other Another useful reagent for reduction of -acetoxyketones and similar compounds
Functional Groups
is samarium diiodide. 233 SmI is a strong one-electron reducing agent, and it is believed
2
that the reductive elimination occurs after a net two-electron reduction of the carbonyl
group.
O O – OH OH OH
SmI + SmI
RCCHR′ 2 RCCHR′ H RCCHR′ 2 RCCHR′ RC CHR′
.
.
–
O CR′′ O CR′′ O CR′′ O CR′′
2
2
2
2
These conditions were used, for example, in the preparation of the anticancer compound
10-deacetoxytaxol.
CH CO 2 O
3
O OH
OH
HO
HO SmI 2
THF HO O O
HO O O
Ph AcO Ph O AcO
O
Ref. 234
Scheme 5.12 gives some examples of the reductive removal of functional groups
adjacent to carbonyl groups. Entry 1 is an application of this reaction as it was used
in an early steroid synthesis. The reaction in Entry 2 utilizes calcium in ammonia
for the reduction. The reaction in Entry 3 converts the acyloin derived from dimethyl
decanedicarboxylate into cyclodecanone. In the reaction in Entry 4, a sulfonate group
is removed. In Entry 5 an epoxide is opened using aluminum amalgam, and in Entry
6 a lactone ring is opened. The latter reaction was part of a synthetic sequence in
which the lactone intermediate was used to establish the stereochemistry of the acyclic
product. The reaction in Entry 7 removes a sulfinyl group. Keto sulfoxides can be
obtained by acylation of the anion of dimethylsulfoxide, so this reaction constitutes a
general route to ketones (see Section 2.3.2). The reaction in Entry 8 is a vinylogous
version of the reduction. The reductant in Entries 9 and 10 is SmI . In Entry 9, the
2
2-phenylcyclohexyloxy group that is removed was used earlier in the synthesis as a
chiral auxiliary. Samarium diiodide is useful for deacetoxylation or dehydroxylation
of -oxygenated lactones derived from carbohydrates (Entry 10). 235 The reaction is
also applicable to protected hydroxy groups, such as in acetonides. The reactions in
Scheme 5.12 include quite a broad range of reductable groups, including some (e.g.,
ether) that are modest leaving groups.
233 G. A. Molander and G. Hahn, J. Org. Chem., 51, 1135 (1986).
234 R. A. Holton, C. Somoza, and K.-B. Chai, Tetrahedron Lett., 35, 1665 (1994).
235
S. Hanessian, C. Girard, and J. L. Chiara, Tetrahedron Lett., 33, 573 (1992).