Page 468 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 468
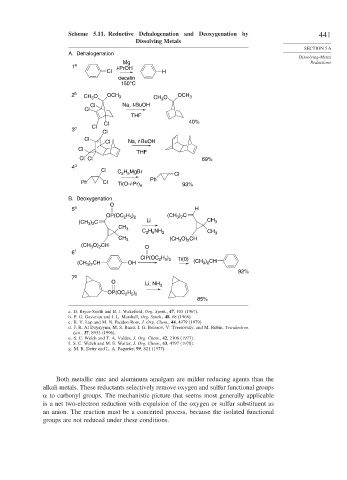
Scheme 5.11. Reductive Dehalogenation and Deoxygenation by 441
Dissolving Metals
SECTION 5.6
A. Dehalogenation
Dissolving-Metal
Mg Reductions
1 a i-PrOH
Cl H
decalin
150°C
2 b O OCH 3 OCH
CH 3 CH O 3
3
Cl Na, t-BuOH
Cl
THF
Cl 40%
3 c Cl
Cl
Cl
Cl Na, t-BuOH
Cl
THF
Cl Cl 69%
4 d
Cl H MgBr
Cl
C 2 5
Ph
Ph Cl Ti(O-i-Pr) 4 93%
B. Deoxygenation
O
5 e H
OP(OC H ) (CH ) C
2 5 2
3 2
(CH ) C Li CH 3
3 2
CH 3
C H NH 2 CH 3
2 5
CH 3 (CH O) CH
2
3
(CH O) CH O
2
3
6 f
ClP(OC H ) Ti(0)
2 5 2
) CH
3 2
(CH 3 2 OH (CH ) CH
92%
7 g
O Li, NH 3
OP(OC H )
2 5 2
85%
a. D. Bryce-Smith and B. J. Wakefield, Org. Synth., 47, 103 (1967).
b. P. G. Gassman and J. L. Marshall, Org. Synth., 48, 68 (1968).
c. B. V. Lap and M. N. Paddon-Row, J. Org. Chem., 44, 4979 (1979).
d. J. R. Al Duyayymi, M. S. Baird, I. G. Bolesov, V. Tversovsky, and M. Rubin, Tetrahedron
Lett., 37, 8933 (1996).
e. S. C. Welch and T. A. Valdes, J. Org. Chem., 42, 2108 (1977).
f. S. C. Welch and M. E. Walter, J. Org. Chem., 43, 4797 (1978).
g. M. R. Detty and L. A. Paquette, 99, 821 (1977).
Both metallic zinc and aluminum amalgam are milder reducing agents than the
alkali metals. These reductants selectively remove oxygen and sulfur functional groups
to carbonyl groups. The mechanistic picture that seems most generally applicable
is a net two-electron reduction with expulsion of the oxygen or sulfur substituent as
an anion. The reaction must be a concerted process, because the isolated functional
groups are not reduced under these conditions.