Page 471 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 471
444 Scheme 5.12. (Continued)
CHAPTER 5 a. R. B. Woodward, F. Sondheimer, D. Taub, K. Heusler, and M. W. McLamore, J. Am. Chem. Soc., 74, 4223 (1952).
b. J. A. Marshall and H. Roebke, J. Org. Chem., 34, 4188 (1969).
Reduction of c. A. C. Cope, J. W. Barthel, and R. D. Smith, Org. Synth., 1V, 218 (1963).
Carbon-Carbon Multiple d. T. Ibuka, K. Hayashi, H. Minakata, and Y. Inubushi, Tetrahedron Lett., 159 (1979).
Bonds, Carbonyl e. E. J. Corey, E. J. Trybulski, L. S. Melvin, Jr., K. C. Nicolaou, J. A. Secrist, R. Lett, P. W. Sheldrake, J. R. Falck,
Groups, and Other D. J. Brunelle, M. F. Haslanger, S. Kim, and S. Yoo, J. Am. Chem. Soc., 100, 4618 (1978).
Functional Groups f. P. A. Grieco, E. Williams, H. Tanaka, and S. Gilman, J. Org. Chem., 45, 3537 (1980).
g. E. J. Corey and M. Chaykovsky, J. Am. Chem. Soc., 86, 1639 (1964).
h. L. E. Overman and C. Fukaya, J. Am. Chem. Soc., 102, 1454 (1980).
i. J. Castro, H. Sorensen, A. Riera, C. Morin, A. Moyano, M. A. Pericas, and A. E. Greene, J. Am. Chem. Soc., 112, 9388
(1990).
j. S. Hanessian, C. Girard, and J. L. Chiara, Tetrahedron Lett., 33, 573 (1992).
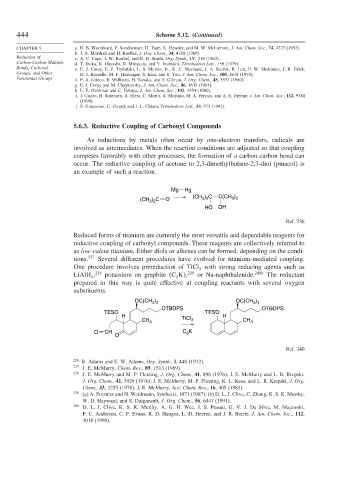
5.6.3. Reductive Coupling of Carbonyl Compounds
As reductions by metals often occur by one-electron transfers, radicals are
involved as intermediates. When the reaction conditions are adjusted so that coupling
competes favorably with other processes, the formation of a carbon-carbon bond can
occur. The reductive coupling of acetone to 2,3-dimethylbutane-2,3-diol (pinacol) is
an example of such a reaction.
Mg Hg
3 2
3 2
(CH ) C O (CH ) C C(CH )
3 2
HO OH
Ref. 236
Reduced forms of titanium are currently the most versatile and dependable reagents for
reductive coupling of carbonyl compounds. These reagents are collectively referred to
as low-valent titanium. Either diols or alkenes can be formed, depending on the condi-
tions. 237 Several different procedures have evolved for titanium-mediated coupling.
One procedure involves prereduction of TiCl with strong reducing agents such as
3
LiAlH , 238 potassium on graphite C K , 239 or Na-naphthalenide. 240b The reductant
4 8
prepared in this way is quite effective at coupling reactants with several oxygen
substituents.
OC(CH ) OC(CH )
3 3
3 3
OTBDPS OTBDPS
TESO TESO
H H
TiCl 3 CH
CH 3 3
O CH C K
8
O
Ref. 240
236
R. Adams and E. W. Adams, Org. Synth., I, 448 (1932).
237 J. E. McMurry, Chem. Rev., 89, 1513 (1989).
238
J. E. McMurry and M. P. Fleming, J. Org. Chem., 41, 896 (1976); J. E. McMurry and L. R. Krepski,
J. Org. Chem., 41, 3929 (1976); J. E. McMurry, M. P. Fleming, K. L. Kees, and L. R. Krepski, J. Org.
Chem., 43, 3255 (1978); J. E. McMurry, Acc. Chem. Res., 16, 405 (1983).
239 (a) A. Furstner and H. Weidmann, Synthesis, 1071 (1987); (b) D. L. J. Clive, C. Zhang, K. S. K. Murthy,
W. D. Hayward, and S. Daigneault, J. Org. Chem., 56, 6447 (1991).
240
D. L. J. Clive, K. S. K. Murthy, A. G. H. Wee, J. S. Prasad, G. V. J. Da Silva, M. Majewski,
P. C. Anderson, C. F. Evans, R. D. Haugen, L. D. Heerze, and J. R. Barrie, J. Am. Chem. Soc., 112,
3018 (1990).