Page 474 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 474
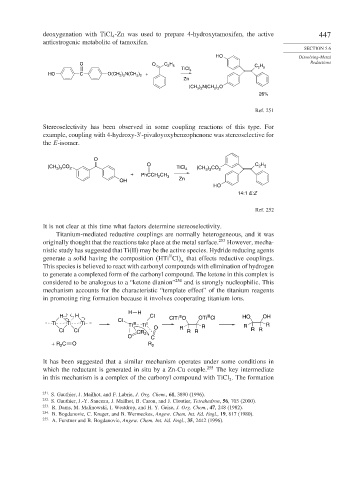
deoxygenation with TiCl -Zn was used to prepare 4-hydroxytamoxifen, the active 447
4
antiestrogenic metabolite of tamoxifen.
SECTION 5.6
HO Dissolving-Metal
O O C H 5 C H Reductions
2
TiCl 4 2 5
HO C O(CH ) N(CH ) +
3 2
2 2
Zn
) N(CH ) O
(CH 3 2
2 2
26%
Ref. 251
Stereoselectivity has been observed in some coupling reactions of this type. For
example, coupling with 4-hydroxy-3 -pivaloyoxybenzophenone was stereoselective for
the E-isomer.
O
O C H
(CH ) CO 2 TiCl 4 (CH ) CO 2 2 5
3 3
3 3
+ PhCCH CH 3
2
OH Zn
HO
14:1 E:Z
Ref. 252
It is not clear at this time what factors determine stereoselectivity.
Titanium-mediated reductive couplings are normally heterogeneous, and it was
originally thought that the reactions take place at the metal surface. 253 However, mecha-
nistic study has suggested that Ti(II) may be the active species. Hydride reducing agents
II
generate a solid having the composition HTi Cl that effects reductive couplings.
n
This species is believed to react with carbonyl compounds with elimination of hydrogen
to generate a complexed form of the carbonyl compound. The ketone in this complex is
considered to be analogous to a “ketone dianion” 254 and is strongly nucleophilic. This
mechanism accounts for the characteristic “template effect” of the titanium reagents
in promoting ring formation because it involves cooperating titanium ions.
H H
H H Cl III OTi Cl HO OH
III
Cl ClTi O
Ti Ti Ti Ti III Ti I R R R
Cl Cl CR O R R R R R
O 2 C
+ R C O R 2
2
It has been suggested that a similar mechanism operates under some conditions in
which the reductant is generated in situ by a Zn-Cu couple. 255 The key intermediate
in this mechanism is a complex of the carbonyl compound with TiCl . The formation
2
251
S. Gauthier, J. Mailhot, and F. Labrie, J. Org. Chem., 61, 3890 (1996).
252
S. Gauthier, J.-Y. Sanceau, J. Mailhot, B. Caron, and J. Cloutier, Tetrahedron, 56, 703 (2000).
253 R. Dams, M. Malinowski, I. Westdrop, and H. Y. Geise, J. Org. Chem., 47, 248 (1982).
254 B. Bogdanovic, C. Kruger, and B. Wermeckes, Angew. Chem. Int. Ed. Engl., 19, 817 (1980).
255
A. Furstner and B. Bogdanovic, Angew. Chem. Int. Ed. Engl., 35, 2442 (1996).