Page 478 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 478
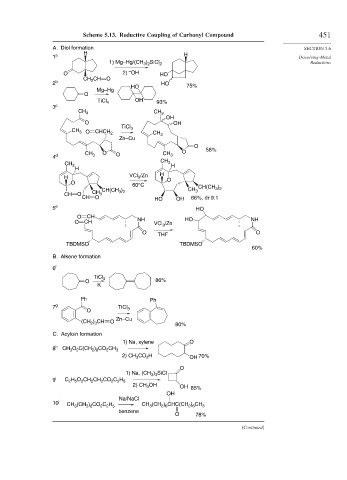
Scheme 5.13. Reductive Coupling of Carbonyl Compound 451
A. Diol formation SECTION 5.6
H H
1 a Dissolving-Metal
1) Mg–Hg/(CH ) SiCl 2 Reductions
3 2
–
O 2) OH HO
CH 2 CH O
2 b HO
HO 75%
Mg–Hg
O
OH
TiCl 4 93%
3 c
CH 3 CH 3
OH
O OH
TiCl 3
CH 3 O CHCH 2 CH
Zn–Cu 3
O
O O O 58%
4 d CH 3 CH 3
CH 3 CH 3 H
H
H VCl 3 /Zn H O
O 60°C CH(CH )
3 2
CH O CH 3 CH(CH ) CH 3 3 2
CH O HO OH 66%, dr 9:1
5 e HO
O CH
O CH NH VCl /Zn HO NH
3
O THF O
TBDMSO TBDMSO
60%
B. Alkene formation
6 f
TiCl 3
O 86%
K
Ph Ph
7 g TiCl 3
O
Zn–Cu
(CH ) CH O 80%
2 3
C. Acyloin formation
1) Na, xylene O
8 h CH 3 O C(CH ) CO CH 3
2
2 8
2
2) CH CO H OH 70%
2
3
O
1) Na, (CH ) SiCl
3 3
9 i C H O CH CH CO C H
2
2 5
2
2
2 2 5
2) CH 3 OH OH 85%
OH
Na/NaCl
10 j CH (CH ) CO C H CH 3 (CH ) CHC(CH ) CH 3
2 6
2 6
2 6
3
2 2 5
benzene
O 78%
(Continued)