Page 482 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 482
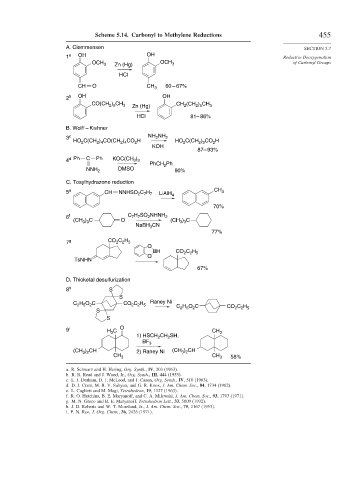
Scheme 5.14. Carbonyl to Methylene Reductions 455
A. Clemmensen SECTION 5.7
1 a OH OH Reductive Deoxygenation
OCH of Carbonyl Groups
Zn (Hg)
OCH 3 3
HCl
CH O CH 3 60 – 67%
2 b OH OH
) CH CH (CH ) CH
CO(CH 2 5 3 Zn (Hg) 2 2 5 3
HCl 81– 86%
B. Wolff – Kishner
2
3 c NH NH 2
HO C(CH ) CO(CH ) CO H HO C(CH ) CO H
2
2 4
2 4
2
2
2 9
2
KOH
87– 93%
4 d Ph C Ph KOC(CH )
3 3
PhCH Ph
2
NNH 2 DMSO 90%
C. Tosylhydrazone reduction
5 e CH NNHSO C H LiAlH 4 CH 3
2 7 7
70%
6 f C H SO NHNH 2
7 7
2
(CH ) C O (CH ) C
3 3
3 3
NaBH CN
3
77%
C H
7 g CO 2 2 5
O
BH CO C H
2 2 5
O
TsNHN
67%
D. Thioketal desulfurization
8 h S
S
C H O C CO C H Raney Ni C H O C
2 2 5
2
2 5
S 2 5 2 CO 2 C 2 H 5
S
9 i C O CH
H 3 3
1) HSCH CH SH,
2
2
BF 3
(CH ) CH 2) Raney Ni (CH ) CH
3 2
3 2
CH 3 CH 3 58%
a. R. Schwarz and H. Hering, Org. Synth., IV, 203 (1963).
b. R. R. Read and J. Wood, Jr., Org. Synth., III, 444 (1955).
c. L. J. Durham, D. J. McLeod, and J. Cason, Org. Synth., IV, 510 (1963).
d. D. J. Cram, M. R. V. Sahyun, and G. R. Knox, J. Am. Chem. Soc., 84, 1734 (1962).
e. L. Caglioti and M. Magi, Tetrahedron, 19, 1127 (1963).
f. R. O. Hutchins, B. E. Maryanoff, and C. A. Milewski, J. Am. Chem. Soc., 93, 1793 (1971).
g. M. N. Greco and B. E. Maryanoff, Tetrahedron Lett., 33, 5009 (1992).
h. J. D. Roberts and W. T. Moreland, Jr., J. Am. Chem. Soc., 75, 2167 (1953).
i. P. N. Rao, J. Org. Chem., 36, 2426 (1971).