Page 484 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 484
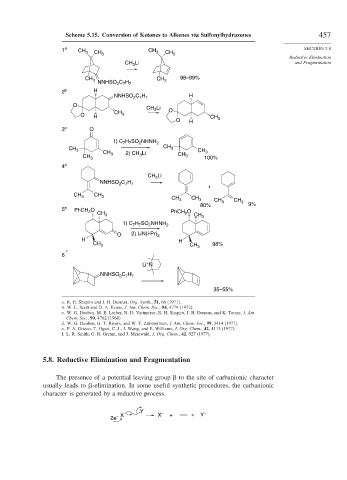
Scheme 5.15. Conversion of Ketones to Alkenes via Sulfonylhydrazones 457
1 a CH 3 CH CH 3 CH SECTION 5.8
3
3
Reductive Elimination
CH Li and Fragmentation
3
CH 3 CH 3 98–99%
NNHSO C H
2 7 7
2 b H
NNHSO C H H
2 7 7
O
CH 3 Li O
CH
O H 3 CH
O H 3
3 c O
H SO NHNH
1) C 7 7 2 2
CH
CH 3 3 CH
CH 2) CH Li 3
CH 3 3 3 CH 3 100%
4 d
CH Li
3
NNHSO C H
2 7 7
+
CH 3 CH 3
CH 3 CH 3 CH 3 CH 2
80% 9%
5 e O
PhCH 2
CH 3 PhCH O CH 3
2
1) C H SO NHNH 2
2
7 7
O 2) LiN(i-Pr) 2
H H
CH 3 CH 3 98%
f
6
+
Li N –
NNHSO C H
2 7 7
35–55%
a. R. H. Shapiro and J. H. Duncan, Org. Synth., 51, 66 (1971).
b. W. L. Scott and D. A. Evans, J. Am. Chem. Soc., 94, 4779 (1972).
c. W. G. Dauben, M. E. Lorber, N. D. Vietmeyer, R. H. Shapiro, J. H. Duncan, and K. Tomer, J. Am.
Chem. Soc., 90, 4762 (1968).
d. W. G. Dauben, G. T. Rivers, and W. T. Zimmerman, J. Am. Chem. Soc., 99, 3414 (1977).
e. P. A. Grieco, T. Oguri, C.-L. J. Wang, and E. Williams, J. Org. Chem., 42, 4113 (1977).
f. L. R. Smith, G. R. Gream, and J. Meinwald, J. Org. Chem., 42, 927 (1977).
5.8. Reductive Elimination and Fragmentation
The presence of a potential leaving group to the site of carbanionic character
usually leads to -elimination. In some useful synthetic procedures, the carbanionic
character is generated by a reductive process.
Y – + Y –
2e – X X +