Page 664 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 664
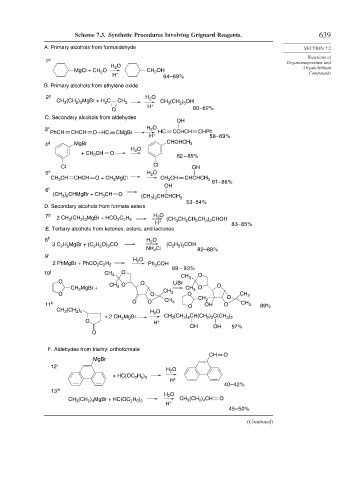
Scheme 7.3. Synthetic Procedures Involving Grignard Reagents. 639
A. Primary alcohols from formaldehyde SECTION 7.2
Reactions of
1 a Organomagnesium and
H O Organolithium
2
MgCl + CH O CH OH
2
2
H + 64–69% Compounds
B. Primary alcohols from ethylene oxide
2 b H O
2
CH (CH ) MgBr + H C CH 2 CH (CH ) OH
2
2 3
3
2 5
3
H +
O 60–62%
C. Secondary alcohols from aldehydes
OH
3 c H O
2
PhCH CHCH O + HC CMgBr HC CCHCH CHPh
H + 58–69%
4 d MgBr CHOHCH 3
O
H 2
CH O
+ CH 3
82 – 85%
Cl Cl OH
5 e H O
2
CH CH CHCH O + CH MgCl CH CH CHCHCH 3
3
3
3
81–86%
OH
6 f
) CHMgBr + CH CH
(CH 3 2 3 O (CH ) CHCHCH 3
3 2
53–54%
D. Secondary alcohols from formate esters
7 g 2 CH (CH ) MgBr + HCO C H H O CH CH CH ) CHOH
2
3
2 3
2 2 5
H + (CH 3 2 2 2 2 83–85%
E. Tertiary alcohols from ketones, esters, and lactones
8 h H O
2
3 C H MgBr + (C H O) CO (C 2 5 3
H ) COH
2 5
2 5
2
NH Cl 82–88%
4
9 i
H O
2 PhMgBr + PhCO C H 2 Ph COH
2 2 5
3
89 – 93%
10 j CH 3 O CH O
O O LiBr 3
MgBr + CH 3 O CH O O
CH 2 CH 3
O O 3 O CH O CH 3
2
11 k O O CH 3 O OH O CH 3 89%
CH (CH ) H 2 O
3
2 4
+ 2 CH MgBr CH (CH ) CH(CH ) C(CH )
3
2 4
3 2
2 2
3
O H +
OH OH 57%
O
F. Aldehydes from triethyl orthoformate
CH O
MgBr
12 l
H O
2
+ HC(OC H )
2 5 3
H +
40–42%
13 m
H 2 O
CH (CH ) MgBr + HC(OC H ) CH 3 (CH ) CH O
2 4
2 4
3
2 5 3
H +
45–50%
(Continued)