Page 667 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 667
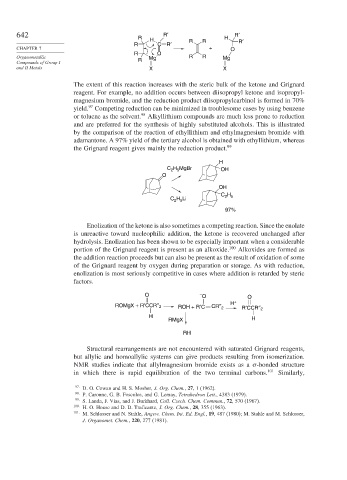
642 R′ R′
R
H R R H R′
R C R′
CHAPTER 7 + O
R O
Organometallic Mg R R Mg
Compounds of Group I R
and II Metals X X
The extent of this reaction increases with the steric bulk of the ketone and Grignard
reagent. For example, no addition occurs between diisopropyl ketone and isopropyl-
magnesium bromide, and the reduction product diisopropylcarbinol is formed in 70%
97
yield. Competing reduction can be minimized in troublesome cases by using benzene
98
or toluene as the solvent. Alkyllithium compounds are much less prone to reduction
and are preferred for the synthesis of highly substituted alcohols. This is illustrated
by the comparison of the reaction of ethyllithium and ethylmagnesium bromide with
adamantone. A 97% yield of the tertiary alcohol is obtained with ethyllithium, whereas
the Grignard reagent gives mainly the reduction product. 99
H
C H MgBr OH
2 5
O
OH
C H
2 5
C H Li
2 5
97%
Enolization of the ketone is also sometimes a competing reaction. Since the enolate
is unreactive toward nucleophilic addition, the ketone is recovered unchanged after
hydrolysis. Enolization has been shown to be especially important when a considerable
portion of the Grignard reagent is present as an alkoxide. 100 Alkoxides are formed as
the addition reaction proceeds but can also be present as the result of oxidation of some
of the Grignard reagent by oxygen during preparation or storage. As with reduction,
enolization is most seriously competitive in cases where addition is retarded by steric
factors.
O – O O
H +
ROMgX + R′CCR″ 2 ROH + R′C CR″ 2 R′CCR″ 2
H
RMgX H
RH
Structural rearrangements are not encountered with saturated Grignard reagents,
but allylic and homoallylic systems can give products resulting from isomerization.
NMR studies indicate that allylmagnesium bromide exists as a -bonded structure
in which there is rapid equilibration of the two terminal carbons. 101 Similarly,
97 D. O. Cowan and H. S. Mosher, J. Org. Chem., 27, 1 (1962).
98 P. Caronne, G. B. Foscolos, and G. Lemay, Tetrahedron Lett., 4383 (1979).
99
S. Landa, J. Vias, and J. Burkhard, Coll. Czech. Chem. Commun., 72, 570 (1967).
100 H. O. House and D. D. Traficante, J. Org. Chem., 28, 355 (1963).
101
M. Schlosser and N. Stahle, Angew. Chem. Int. Ed. Engl., 19, 487 (1980); M. Stahle and M. Schlosser,
J. Organomet. Chem., 220, 277 (1981).