Page 735 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 735
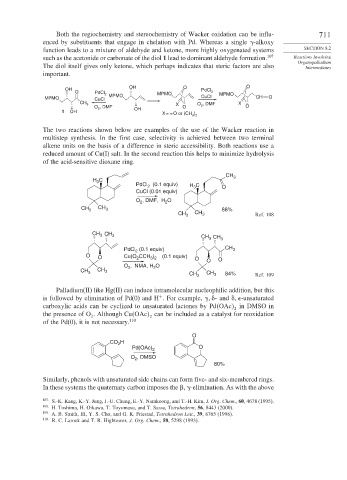
Both the regiochemistry and stereochemistry of Wacker oxidation can be influ- 711
enced by substituents that engage in chelation with Pd. Whereas a single -alkoxy
function leads to a mixture of aldehyde and ketone, more highly oxygenated systems SECTION 8.2
such as the acetonide or carbonate of the diol 1 lead to dominant aldehyde formation. 107 Reactions Involving
Organopalladium
The diol itself gives only ketone, which perhaps indicates that steric factors are also Intermediates
important.
OH O O
OH PdCl
O PdCl 2 MPMO 2 MPMO
MPMO CuCl MPMO CuCl CH O
CH 3 X O , DMF X
O , DMF OH O 2 O
2
1 OH
X = = O or (CH 3 ) 2
The two reactions shown below are examples of the use of the Wacker reaction in
multistep synthesis. In the first case, selectivity is achieved between two terminal
alkene units on the basis of a difference in steric accessibility. Both reactions use a
reduced amount of Cu(I) salt. In the second reaction this helps to minimize hydrolysis
of the acid-sensitive dioxane ring.
CH 3
H C
3
PdCl (0.1 equiv) H C O
2
3
CuCl (0.01 equiv)
, DMF, H O
O 2 2
CH 3 CH 3 88%
CH 3 CH 3 Ref. 108
CH 3 CH 3 CH 3 CH 3
PdCl (0.1 equiv) CH 3
2
O O Cu(O CCH ) (0.1 equiv) O O
2
3 2
, NMA, H O O
O 2 2
CH
CH 3 3 CH
CH 3 3 84% Ref. 109
Palladium(II) like Hg(II) can induce intramolecular nucleophilic addition, but this
is followed by elimination of Pd(0) and H . For example,
- and
-unsaturated
+
carboxylic acids can be cyclized to unsaturated lactones by Pd OAc in DMSO in
2
the presence of O . Although Cu OAc can be included as a catalyst for reoxidation
2
2
of the Pd(0), it is not necessary. 110
O
CO H
2
Pd(OAc) 2 O
, DMSO
O 2
80%
Similarly, phenols with unsaturated side chains can form five- and six-membered rings.
In these systems the quaternary carbon imposes the -elimination. As with the above
107
S.-K. Kang, K.-Y. Jung, J.-U. Chung, E.-Y. Namkoong, and T.-H. Kim, J. Org. Chem., 60, 4678 (1995).
108
H. Toshima, H. Oikawa, T. Toyomasu, and T. Sassa, Tetrahedron, 56, 8443 (2000).
109 A. B. Smith, III, Y. S. Cho, and G. K. Friestad, Tetrahedron Lett., 39, 8765 (1998).
110
R. C. Larock and T. R. Hightower, J. Org. Chem., 58, 5298 (1993).