Page 191 - Advanced Thermodynamics for Engineers, Second Edition
P. 191
178 CHAPTER 9 THERMODYNAMIC PROPERTIES OF IDEAL GASES
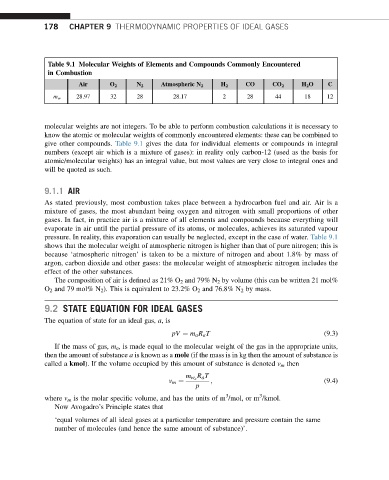
Table 9.1 Molecular Weights of Elements and Compounds Commonly Encountered
in Combustion
Air O 2 N 2 Atmospheric N 2 H 2 CO CO 2 H 2 O C
28.97 32 28 28.17 2 28 44 18 12
m w
molecular weights are not integers. To be able to perform combustion calculations it is necessary to
know the atomic or molecular weights of commonly encountered elements: these can be combined to
give other compounds. Table 9.1 gives the data for individual elements or compounds in integral
numbers (except air which is a mixture of gases): in reality only carbon-12 (used as the basis for
atomic/molecular weights) has an integral value, but most values are very close to integral ones and
will be quoted as such.
9.1.1 AIR
As stated previously, most combustion takes place between a hydrocarbon fuel and air. Air is a
mixture of gases, the most abundant being oxygen and nitrogen with small proportions of other
gases. In fact, in practice air is a mixture of all elements and compounds because everything will
evaporate in air until the partial pressure of its atoms, or molecules, achieves its saturated vapour
pressure. In reality, this evaporation can usually be neglected, except in the case of water. Table 9.1
shows that the molecular weight of atmospheric nitrogen is higher than that of pure nitrogen; this is
because ‘atmospheric nitrogen’ is taken to be a mixture of nitrogen and about 1.8% by mass of
argon, carbon dioxide and other gases: the molecular weight of atmospheric nitrogen includes the
effect of the other substances.
The composition of air is defined as 21% O 2 and 79% N 2 by volume (this can be written 21 mol%
O 2 and 79 mol% N 2 ). This is equivalent to 23.2% O 2 and 76.8% N 2 by mass.
9.2 STATE EQUATION FOR IDEAL GASES
The equation of state for an ideal gas, a,is
pV ¼ m a R a T (9.3)
If the mass of gas, m a , is made equal to the molecular weight of the gas in the appropriate units,
then the amount of substance a is known as a mole (if the mass is in kg then the amount of substance is
called a kmol). If the volume occupied by this amount of substance is denoted v m then
m w a a T
R
v m ¼ ; (9.4)
p
3
3
where v m is the molar specific volume, and has the units of m /mol, or m /kmol.
Now Avogadro’s Principle states that
‘equal volumes of all ideal gases at a particular temperature and pressure contain the same
number of molecules (and hence the same amount of substance)’.